Baum karfe 99,9%
Breif GabatarwanaZaman jama'aKarfe Granules:
Sunan Samfurin: Barum Karfe Granules
CAS: 7440-39-39
Tsarkake: 99.9%
Formudu: Ba
Girman: -20mm, 20-50mm (a ƙarƙashin man ma'adinai)
Maɗaukaki: 725 ° C (lit.)
Tafasa misali: 1640 ° C (lit.)
Yawan: 3.6 g / ml a 25 ° C (lit.)
Temple Tempt. yankin-kyauta
Form: sanduna, chunks, granules
Takamaiman nauyi: 3.51
Launi: azurfa-launin toka
Regise: 50.0 μω-cm, 20 ° C
Bariium wani yanki ne na sinadarai tare da alamar lamba 56. Yana da kashi na biyar a cikin rukuni na 2, wani ƙarfe alkaline ƙasa ƙarfe. Saboda babban ha'inci mai guba, ba a samun barum a cikin yanayi a matsayin mai kyauta. Hydroxide, da aka sani a tarihin zamani kamar yadda Barryta, ba ya faruwa a matsayin ma'adinai, amma ana iya shirya ta hanyar dumama ta Carbonate.
Aikace-aikace: Karfe da allos, suna ɗaukar alluna; Aljihunan sayar da kayayyaki - don ƙara yawan juriya na Creep; Alloy tare da nickel don toshe matastoci; da yawa ga karfe da kuma jefa baƙin ƙarfe a matsayin inoculant; Alloys tare da allium, manganese, silicon, da aluminum a matsayin manyan-aji mai dauke da karfe.Bariium yana da 'yan aikace-aikace na masana'antu. An yi amfani da ƙarfe na ƙarfe don tsage iska a cikin shambura. Yana da bangaren YBCO (manyan-zazzabi na Superconductucntuctuctuction) da silili na lantarki, kuma an ƙara ƙarfe don rage girman hatsi na carbon a cikin microsructure na ƙarfe.
Barium, a matsayin ƙarfe ko lokacin da rigeded tare da aluminium, ana amfani dashi don cire gas da aka so (guttering) daga shubsu na gida. Bariium ya dace da wannan dalili saboda matsanancin matsin wuta da kuma amsawa ga oxygen, nitrogen, carbon dioxide, da ruwa; Zai iya zama har ma a cikin gasalan gas mai kyau ta hanyar narkar da su a cikin lattice. Wannan aikace-aikacen yana ɓacewa ne a hankali saboda tasirin saukar da wutar lantarki da kuma plasma.
Barium, a matsayin ƙarfe ko lokacin da rigeded tare da aluminium, ana amfani dashi don cire gas da aka so (guttering) daga shubsu na gida. Bariium ya dace da wannan dalili saboda matsanancin matsin wuta da kuma amsawa ga oxygen, nitrogen, carbon dioxide, da ruwa; Zai iya zama har ma a cikin gasalan gas mai kyau ta hanyar narkar da su a cikin lattice. Wannan aikace-aikacen yana ɓacewa ne a hankali saboda tasirin saukar da wutar lantarki da kuma plasma.
Coa na baraium karfe granules



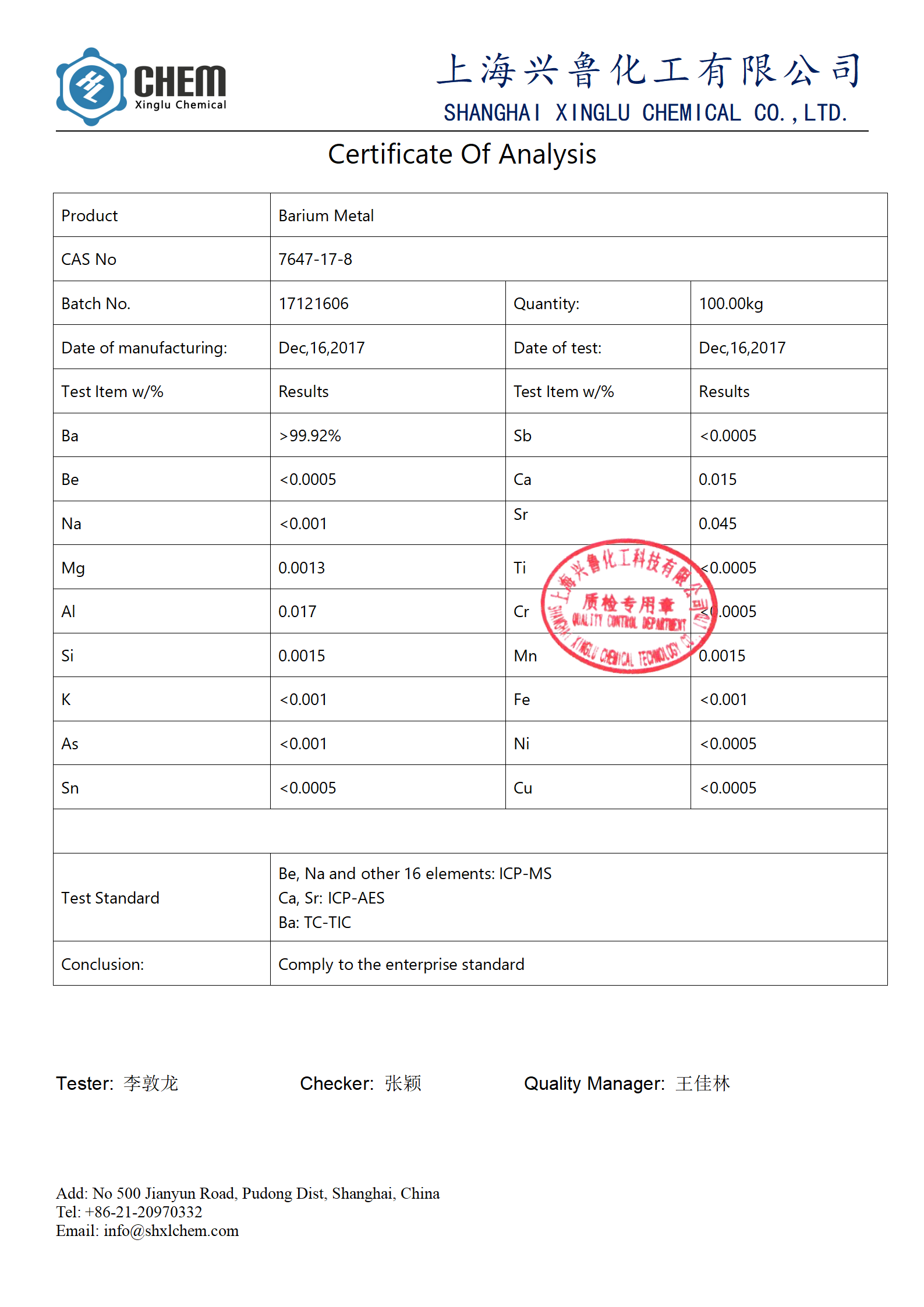
 Abin da za mu iya bayarwa:
Abin da za mu iya bayarwa: