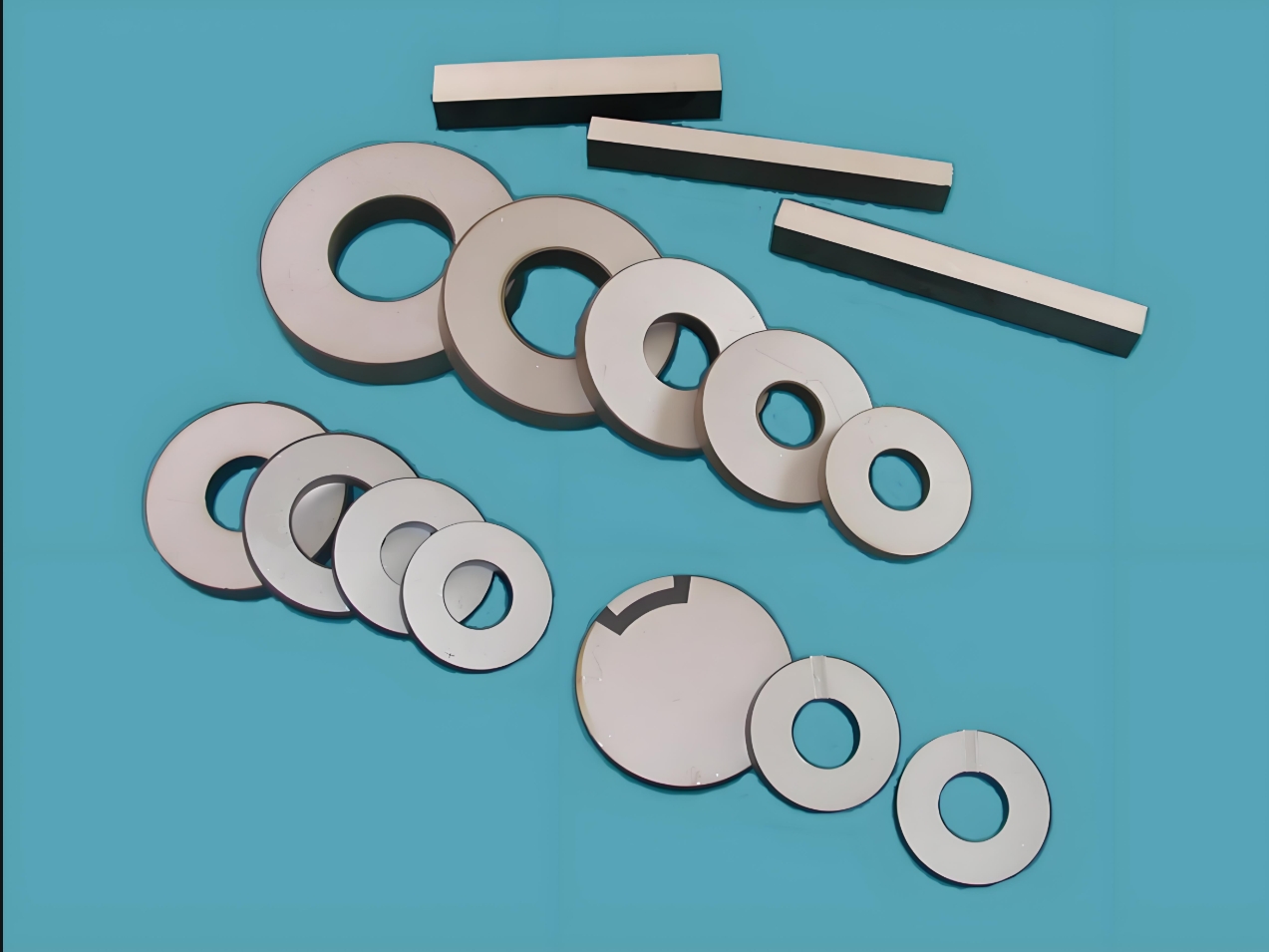
 Abubuwa na Duniyalokaci ne na gaba daya tsawon abubuwa na karfe 17, gami da abubuwan 15 na Lanthani datsirar da ruwadayttrium. Tun daga ƙarshen karni na 18, an yi amfani da su sosai a cikin ƙarfe, Gilerics, Fetracheemicals, bugu, bugun jini, bugun jini da kuma dyeing, noma da kuma sauran masana'antu. Aikace-aikacen da ƙarancin duniya da yawa a masana'antar Ceramicas na fara a cikin 1930s. A cikin 1970s, jimlar adadinm duniyaAnyi amfani da shi a cikin kayan yakan kai 70t / shekara, lissafin kusan 2% zuwa 3% na yawan samarwa na cikin gida. A halin yanzu, ana amfani da ƙasa da yawa a cikin tsarin ramuka na tsarin hatsi, rererication na aiki, yumbu mai haske da sauran filayen. Tare da cigaban ci gaba da aikace-aikacen sababbin kayan duniya masu wuya, ana amfani da ƙasa mai wuya azaman kayan aikinsu, yana inganta farashin kayan aiki, kuma yana sa aikace-shirye su ke yiwuwa.
Abubuwa na Duniyalokaci ne na gaba daya tsawon abubuwa na karfe 17, gami da abubuwan 15 na Lanthani datsirar da ruwadayttrium. Tun daga ƙarshen karni na 18, an yi amfani da su sosai a cikin ƙarfe, Gilerics, Fetracheemicals, bugu, bugun jini, bugun jini da kuma dyeing, noma da kuma sauran masana'antu. Aikace-aikacen da ƙarancin duniya da yawa a masana'antar Ceramicas na fara a cikin 1930s. A cikin 1970s, jimlar adadinm duniyaAnyi amfani da shi a cikin kayan yakan kai 70t / shekara, lissafin kusan 2% zuwa 3% na yawan samarwa na cikin gida. A halin yanzu, ana amfani da ƙasa da yawa a cikin tsarin ramuka na tsarin hatsi, rererication na aiki, yumbu mai haske da sauran filayen. Tare da cigaban ci gaba da aikace-aikacen sababbin kayan duniya masu wuya, ana amfani da ƙasa mai wuya azaman kayan aikinsu, yana inganta farashin kayan aiki, kuma yana sa aikace-shirye su ke yiwuwa.
Aikace-aikacen duniya da yawa a cikin ramin ramin
A aikace-aikace aAl2o3Geramics Al2o3 Brerorics sune mafi yawan amfani da beramin berolics saboda ƙarfin ƙarfinsu, rufincar mai kyau, da juriya na lalata, da kyawawan kaddarorin lantarki, da kuma kyawawan kaddarorin lantarki, da kyawawan kaddarorin lantarki, da kyawawan kaddarorin lantarki, da kuma kyawawan kaddarorin lantarki, da kuma kyawawan kaddarorin lantarki, da kuma kyawawan kaddarorin lantarki, da kyawawan kaddarorin lantarki, da kyawawan kaddarorin lantarki, da kyawawan kaddarorin lantarki. Ƙara ƙasa ƙasa ƙasa okides kamarY2o3, Lage3, Sm2o3, da sauransu za a iya inganta kayan wetting kayan kayan kwalliya na Al2O3, rage girman kayan yumɓu; Rage kwanciyar hankali na kayan da ƙara yawan yawa; Hatsa hijirarsu na wasu ions, rage yawan ƙaura na hatsi iyakoki, kuma yana iya sauƙaƙe samuwar ginannun ƙananan; Inganta karfin gilashin, ta hanyar cimma manufar inganta kaddarorin kayan aikin Al2o3.
A aikace-aikace aSi3n4Geramicssi3n4 Nerarmic suna da kyakkyawan kaddarorin kayan aikin injiniyoyi, kadayyarori da kwanciyar hankali, kuma sune kayan kayan kwalliya don berormation na babban-zazzabi. Tunda si3n4 ƙaƙƙarfan haɗin gwiwar haɗin kai ne, tsarkakakken si3n4 ba za a iya yayyafa shi ta hanyar tsarin masarufi na al'ada ba. Sabili da haka, ban da amsawar alamar kai tsaye na si foda, dole ne a ƙara wasu adadin taimakon da ake amfani da shi don yin abu mai yawa. A halin yanzu, mafi kyawun kayan taimako na kayan taimako don shirya Si3n4 cirerication ne mai wuya a cikin ownesY2o3, Nd2o3, daLage3. A gefe guda, waɗannan kwanton ƙasa ƙasa ownides suna amsawa da alama Sioce matakai, wanda yadda ya kamata ya inganta yanayin gilashin Si3n4; A gefe guda, suna samar da iyakokin hatsi na gilashin da ke tattare da haɓakar ƙwayar cuta da yawa, kuma suna da sauƙin tattare da haɓakar ƙarancin zafin jiki na kayan aiki.
A aikace-aikace aZRO2Tererics Zro2 Zero2 Gindige yana da babban yawa, melting Point da wahala, musamman tazara mai ƙarfi da ƙarfi, waɗanda sune mafi girma a cikin dukkanin rerorics. Tun lokacin da canjin Crystal Canjin Zro2 yana tare da canjin bayyana, ikon amfani da kai tsaye yana da iyaka. Tare da zurfafa aikin bincike, ana gano cewa Bugu da ƙari na ƙarancin ƙasa duniya yana da mafi kyawun rigakafi da haɓaka sakamako akan canjin lokaci na Zro2. Wanda aka saba amfani da shi a cikin okes galibiY2o3,Nd2o3, da kuma I2O3. Su ionic radius kusa da na ZR4 +, kuma suna iya samar da monoclinic, tetragonal da kuma strawal ya maye gurbin mafita tare da Zro2. Wannan nau'in kayan yumbu na Zropp yana da kyawawan manufofin fasaha na fasaha. Misali,Ceo2na iya samar da yankin tarar tetragonal Zischonia m a kewayon kewayon da Zro2, wanda shine kyakkyawan abu mai kyau na electrolyte. Y2O3-Stabbized Zro2 (YSZ) kyakkyawan onence ion mai daɗaɗɗen ointenan ointenan opide (sofc), na'urori masu amfani da iskar oxidgation, kuma methane partation na iskar shaye shaye, da kuma methane partation ethrest reactors.
A aikace-aikace aNa SICramusCarbide SiliconGererics suna da tsayayya ga babban yanayin zafi, girgizar zafi, lalata, sutura mai kyau da kuma nauyi mai haske, kuma ana amfani da su yawanci ana amfani da berormics mai haske. Da karfi da hadin kai halaye naNa SICKayyade cewa yana da wuya a cimma nasarar yin amfani da yanayin sa a ƙarƙashin yanayin al'ada. Yawancin lokaci ya zama dole don ƙara saƙonni na yin saƙo ko amfani da matsi mai zafi da zafi maƙaryaci mai zafi. Tsarin samarwa yana da rikitarwa kuma farashin yana da girma. Taimako mai tasiri na taimako na nuna rashin aiki na SIC shine Al2o3; Sic-yag yumbu na damfara tare da ypal5o12 (yag
A aikace-aikace aAlnramusAlnWani babban haɗin kai ne tare da melting aya, babban aiki da kuma juriya ga lalata metals da allura kamar baƙin ƙarfe da aluminum. Yana da kyakkyawan zazzabi babban zazzabi a cikin yanayi na musamman kuma shine ingantaccen sikelin da aka haɗa shi mai ɗorewa da kuma kayan haɗi. Tunda AlN wani abu ne mai covalent, suna da wuya, da taimako guda ɗaya na iya amfani da cutar kanjamau da kuma kayan kwalliya. Bugu da kari, cutar kanjama ta saƙa kuma iya amsawa tare da kayatarwar oxygen a cikiAln, Rage guraben aluminum lalacewa ta hanyar m partygen narkewa cikin Aln lattice a cikin AlN lettice, da kuma inganta halayen da ke da zafin jiki naAln.
■ Aikace-aikace a Sialon tsirar Sialon Sialon Brerich sune irin na Si-ba mai yawan gaske polycrystalline da aka inganta akan lamarinSi3n4Brororics. An kafa su ta hanyar canji na Si atoms da n n atoms inSi3n4by Al atoms da o atoms a cikin Al2oms. Karfinsu, tauri, da juriya na rigakafi sun fi braridation na Si3n4, kuma musamman sun dace da kayayyakin yumbu da samfuran yumbu mai tsayayya da siyarwa. Kayan Sallon ba su da sauƙin sukar. Gabatarwar da ƙarancin ƙasa ta oxtelie ne ke dacewa da samuwar ruwa a ƙananan zafin jiki, wanda ya inganta nuna girman kai. A lokaci guda, karar ƙasa cations na iya shigar da lattice lokaci na α-siuntin lokaci, rage abun ciki na gilashi da kuma inganta yanayin zafin jiki da kuma yawan zafin jiki na kayan aiki na kayan. Nazarin sun nuna cewa ƙara 1%Y2o3Zai iya samar da gilashin gilashi mai tsayi lokacin da Sialon Gilerication a yanayin zafi, wanda ba wai kawai yana inganta suna zama ba, har ma yana inganta tazara. Bugu da kari, ƙara karamin adadin Y2O3 kuma yana inganta juriya da iskar shaka.
Aikace-aikacen duniya da yawa a cikin aikin berammens
M duniyasuna da alaƙa da tsararren ƙwayoyin halitta. Dingara wasuAbubuwa na DuniyaZuwa ga albarkatun ƙasa na ramukan ramukan rudanta da yawa ba kawai zai iya inganta zama da yawa ba, ba kawai iya inganta rikicewar su ba.
1Matsayi a Sofin Cikin Surangare Ranar Bermens tun 1987, lokacin da masana kimiyyar kayan kimiyya daga China daga China, Japan, Amurka da wasu ƙasashe suka gano cewa Bratin HamarideYttrium Barum Cook Oxide(YBCO) suna da kyau m high-zazzabi SuperCaConturity (TC har zuwa 92k), mutane sun yi aiki mai yawa a cikin binciken babban aiki na duniya suna ci gaba, kuma sun sami ci gaba mai yawa. Karatun Japan sun nuna cewa bayan ya maye gurbin y a cikin YBCO tare daHaske mai haske(LN) kamarNd, Sm, Eu, daGd, mawuyacin filin karfin da suka haifar da sakamakon ingantaccen tsarin yumɓu LNBCO ne kuma inganta karfi a cikin wutar lantarki, adana makamashi da sufuri. Jami'ar Peking da aka yi amfani da itaZRO2a matsayin mai substrate kuma yana mai da shi ga kusan 200 ° C, da kuma fitar da y (ko wasum duniya), Ba Oxides da Cu a kan subers a cikin yadudduka don maganin magani, da zafi bi da su a cikin yawan zafin jiki kewayon 800-900 ° C. Sakamakon Superconding Remorcion frifs ya nuna kyakkyawan yanayin zafin jiki na sama da 100k. Jami'ar Kagoshira a Japan ta karaRasa DuniyaLA zuwa SR da NB Oxides don yin fim ɗin yumbu, wanda ya nuna SuperCont At 255k.
2 Aikace-aikacen Aikace-aikacen Piezoelectric cerorics na Titanate (Pbtio3) Shin wani nau'in Piezoeclectrance yumbu ne tare da sakamako mai amfani da makamashin lantarki. Yana da babban zazzabi mai zurfi (490 ° C) da ƙarancin yanke shawara koyaushe, kuma ya dace don aikace-aikace a ƙarƙashin babban zazzabi da yanayin mitar. Koyaya, a lokacin shirye-shiryen sa da sanyin sanyaya, fasahar micro suna haifar da faruwa saboda canjin lokaci-lokaci na Tetragonal. Don magance wannan matsalar, ana amfani da ƙasan duniya mai wuya don canza shi. Bayan sukar da karfe 1150 ° C, sake samun gereran sirerin tare da yawan dangi na 99% za'a iya samu. An inganta inganta makamai kuma ana iya amfani dashi don ƙera hanyoyin fassara mai fassara aiki a ƙarƙashin yanayin mitar na 75mhz. A cikin jagorantar titanate (pzt) Pizoelectcric Brerorics tare da manyan Piezoeclectrric Chapercrics, ta ƙara ƙarancin ƙasa ƙasa da yawa kamarLage3, Sm2o3, daNd2o3, kaddarorin da aka yi na rumman na PZT Brurikation na PZT Brurikai za a iya inganta su da ingantaccen ingancin lantarki da kuma za a iya samu kaddarorin kadarorin lantarki da kaddarorin. Bugu da kari, wasan kwaikwayon Pzt Bramication na iya inganta ta hanyar ƙara karamin adadin ƙasa na ƙasa da yawa a cikin oxideCeo2. Bayan ƙara jagorar czt, tsararren Pzt Bruristicer yana ƙaruwa, wanda ke da dacewa a tabbatar da pantersization a ƙarƙashin babban zazzabi da kuma juriya kan lokacin tsufa da tsufa kuma ana inganta lokacin tsufa da zafin jiki. Pzt tiron gyaranm duniyaAn yi amfani da su sosai a cikin masana'antun masu ƙarfin lantarki, masu samar da masana'antu na ultrasonic, waɗanda ke ƙasa da asarar ruwa da kuma wasu na'urori.
3Aikace-aikace a cikin yurkanni yttrium-kaji Zircin baki (YSZ) Bramics tare daRare CREAS CRINE OXID Y2o3Kamar yadda ƙari da ƙara kyau da kwanciyar hankali da kwanciyar hankali a babban yanayin zafi, kyawawan IPygen Ion da ke cikin OION A CIKIN IION IION CIONRICS. Anyi amfani da nasarar samar da nutsuwa wajen auna matsin oxygen a cikin ababen hawa na iskar oxygen a cikin shaye-shaye, yana sarrafa iska / man fetur sosai, kuma suna da tasirin saiti. An yi amfani da su sosai a cikin manyan masu fasahar masana'antu, masu sihiri, masu cin amana da sauran kayan aiki. Duk da haka, YSZ Bramich Nuna babban ionic halin lokacin da zazzabi ya fi 900 ° C, don haka aikace-aikacen su har yanzu suna batun wasu ƙuntatawa. Binciken da ya kasance ya gano cewa ƙara adadin ya dace da Y2O3 koGd2o3 to Bi2o3Brerormation tare da mafi girma OILINALILALAY CIGABA DA CIKIN SAUKI NA BI2O3 fuska zuwa zazzabi a dakin. At the same time, X-ray diffraction patterns have also shown that (Bi2O3)0.75·(Y2O3)0.25 and (Bi2O3)0.65·(Gd2O3)0.35 are both stable face-centered cubic structures with high oxygen ion conductivity. Bayan yana da gefen wannan yumbu tare da fim mai kariya na (Zro2) 0.92 (y2o3) 0.0 ~ 8) ana iya shirya shi a ƙarƙashin matsalolin da aka haifeshi da manyan fasahar da aka kawo.
4 Aikace-aikacen Canelecrics Secolric Relorrics ana amfani da shi ne musamman don yin ceramic capacitors da abubuwan da aka gyara na obin na lantarki. A cikin secterric ceramics kamarTiO2, Mgtio3,Batoo3da kuma hadewar sittins, ƙaram duniyakamar LA, ND, kuma Dan kuma yana iya inganta kadarorinsu na gyara. Misali, a cikin Batio3 Reramics tare da babban mawuyaci akai-akai, kara kaskantar da LA da ND Rare Cin Matsayi na Daidai, kuma rayuwar sabis na na'urar tana iya inganta sosai. A cikin Meneelecrric Hamors masu ɗaukar hoto, duniya baki daya da ake samu kamar yadda ake buƙata don inganta ko daidaita madaidaicin yanke shawara, da kuma ingantaccen ingancin Gidantarwa, don haka fadada kewayon aikace-aikacen sa. A hankali tsayayyen karfin titan an canza shi da La2o3, da kuma Catio3 na Tioamics ba wai kawai inganta allurar su baakai-akai.
5 Aikace-aikacen Commicitived Coramics Mai haƙuri da Brurication muhimmin nau'i ne mai mahimmanci na aikin rererics mai aiki. An san su ta hanyar yin la'akari da wasu yanayi na waje kamar wutar gas, zazzabi, zafi, da sauransu sabili da haka, suna iya sa ido kan sigogi masu amfani da su ko kuma canza su sigogi masu amfani da wutar lantarki. Ana amfani dasu azaman abubuwan lura da abubuwa a cikin da'irar da'irori, don haka ana kiran su firam ɗin firam. Akwai kusanci tsakanin ƙasan duniya da kuma aikin wannan nau'in garin Bram.
(1) rerorical reramics: ta hanyar ƙara kasada a cikin ƙasa daban-dabanLage3Zuwa pzt, ba da gaskiya na Lanthanum zircontas titanate (plzt) za a iya samu ramili na lantarki na lantarki na lantarki. Ainihin Matrix Pzt gaba daya ne opaque saboda kasancewar pores, da yawa na rage haske da yawa a kan iyakokin hatsi da haske wanda ya haifar da na biyu. Saboda haka, plzt yana da wasan watsa haske mai kyau. An yi amfani da PLZT sosai a cikin Gaggles don kare garkuwar fashewar fashewar Nuclear, Windows Boms, Optical Modulics, na'urorin sadarwa na Hololraphic, da sauransu.
(2) bambance bambance rabban ƙasa: Jami'ar Kudancin Kudu ta Tsakiya ta yi nazarin tasirin abubuwan duniya akan kaddarorin Zno girma. Bayan Zno Girlistor Brarorics an dibs tare da rasanta ƙasaLage3, darajar su ta hanyar su ta hanyar ƙimar VLMA ta karu sosai; Lokacin da adadin doping ya karu daga 0.1% zuwa 10%, madaidaicin madaidaicin madaidaicin α na yumɓu ya ragu daga 20 zuwa 1, kuma m ba shi da bambance bambance. Saboda haka, don Zno Brorolics, maida hankali ne mai ƙarancin ƙasa na ƙasa da dama na ci gaba, amma ba shi da ƙarfi akan ikon da ba shi da inganci; da kuma taro mai yawa-wuri baya nuna halayen bambance bambancen.
(3) Gas din Gas. Tun daga shekarun 1970, mutane sun yi bincike da yawa a kan kara karatuttukan farko kamar zno,Sno2daFe2O3, kuma sun samar da abo3 da A2bo4 R Speoparancin Duniya suna haɗa kayan Oxigaide. Sakamakon bincike yana nuna cewa ƙara da akasari ƙasa ƙasa da yawa zuwa Zno na iya inganta tunaninta da propylene; ƙaraCeo2ga sno2 na iya samar da kayan da aka yi da shi wanda yake kula da ethanol.
(4) Gleristor Bramication: Barii Titanate (Bario3) shine mafi yawan karatu da kuma yin amfani da Hermistor Brerryor. Lokacin da alama ba ta da yawa daga cikin LA, A., SM, da sauransu cajin caji ta hanyar aiwatar da ruwa a cikin aikin ti4 +, saboda haka ne aka rage ɓangaren yumbu. Koyaya, idan adadin doping ya wuce takamaiman darajar, saboda samuwar Ba2 + + + vacanies da bacewar yadudduka ya tashi sosai kuma har ma ya zama insulator.
(5) Brorormics mai zafi-mai hankali: Daga cikin nau'ikan ramuka daban-daban, ƙasan ƙasa da aka ƙara a halin yanzu ana ƙara yawancin tsarin, kamar tsarin SR1-XLOO3-TOI2-V2OO5 tsarin, SR0.95La0.0,5sno3 da Pd0.91LA0.09 (ZR0.65TI0.35) 0.35) 0.9883-KH2PO3, da dai sauransu domin inganta aikin ci gaba da kwanciyar hankali, kuma wajibi ne don ƙarfafa bincike kan tasirin daRasa Duniyabugu da ƙari a kan abubuwan da suka dace na Bramics.
Mun kware a fitarwa a cikin samfuran ƙasa masu wuya, don siyan kasan duniya mai wuya, barka da zuwaKYAUTATA US
Sales@shxlchem.com; Delia@shxlchem.com
WhatsApp & Tel: 00861352423; 0086 13661632459
Lokacin Post: Feb-06-2025