Ceo2muhimmin bangare ne na kayan duniya masu wuya. DaRare Duniya ceriumYana da tsarin lantarki na musamman na waje - 4F15D16s2. Layer na musamman 4f Layer zai iya adana abubuwa da inganci, yin cerium + na jihar + 3 da + 4 na jihar. Sabili da haka, kayan hannun jari2 suna da ƙarin ramukan oxygen, kuma suna da kyakkyawar ikon adana oxygen. Canjin CE (III) da CE (iv) kuma yana ba da ƙarshen kayan abu tare da abubuwan da ke cikin cataltato na raguwa. Idan aka kwatanta da kayan Bulk, Nano Shugaba, a matsayin sabon nau'in kayan inorganic, ya sami kulawa sosai saboda ikon iskar ophygen, da kuma yawan aikin oxygen, da kuma yawan zafin jiki na oxygen. A halin yanzu akwai yawan rahotannin bincike da aikace-aikace masu alaƙa ta amfani da Kamfanin Nano2 a matsayin mai kunnawa, masu ɗaukar kaya ko ƙari, da adsorbents, da adasorbents.
1. Shiri Hanyar Nanometerberiide reide
A halin yanzu, hanyar shiri gama gari don Nano Ceria ya hada da hanyar sunadarai da hanyar zahiri. Dangane da hanyoyin sunadarai daban-daban, hanyoyin sunadarai za su iya shiga hanyar hazo, hanyar hydrothermal, hanyar solomothermal, hanyar solvothermal, hanyar micronmulsion, hanyar micromulsion, sol mashin da hanyar lantarki; Hanyar ta zahiri ita ce mafi girma hanyar.
1.1 nika hanyar
The grinding method for preparing nano ceria generally uses sand grinding, which has the advantages of low cost, environmental friendliness, fast processing speed, and strong processing ability. A halin yanzu shine mafi mahimmancin aiki a masana'antar Nano Ceria. Misali, shirye-shiryen Nano Ceriin Polishing foda gabaɗaya yana kuma gauraye don maganin pre-cerium. Ta amfani da yashi mai girma iri daban-daban grinding bead ragon, Nano Ceria tare da D50 jere daga dubun zuwa duburrukan nanometers ta hanyar daidaitawa.
1.2 hazo hanya
Hanyar hazo tana nufin hanyar shirya m foda ta hanyar hazo, rabuwa, bushewa, da kuma lissafin albarkatun albarkatun da aka narke a cikin abubuwan da suka dace. Hanyar hazo a yi amfani da ita sosai a cikin shiri na duniya da kuma fa'idoji kamar tsari mai sauƙi, babban aiki, da ƙarancin farashi. Hanyar da aka saba amfani da ita ce don shirya Nano Ceria da kayan haɗin gwiwa a masana'antar. Wannan hanyar na iya shirya Nano Ceria tare da ilimin halittar jiki daban-daban da kuma girman ph, da sauransu hanyoyin dogaro da lalata vions ta hanyar irea. A madadin haka, ana iya haifar da cerium ions preciply da hydrolysis na sodium cirrate, sannan kuma infubated da ceri ceriajers.
1.3 hydrothermal da solvothermal hanyoyin
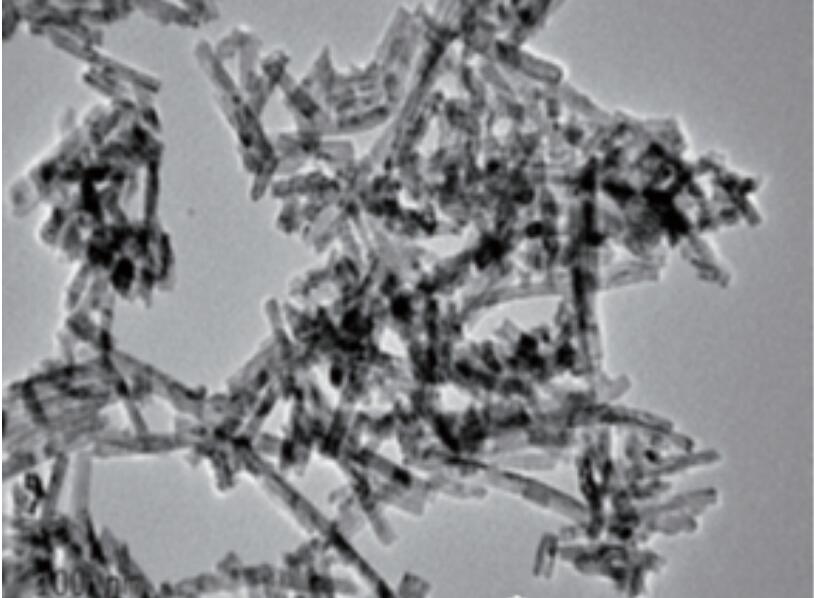
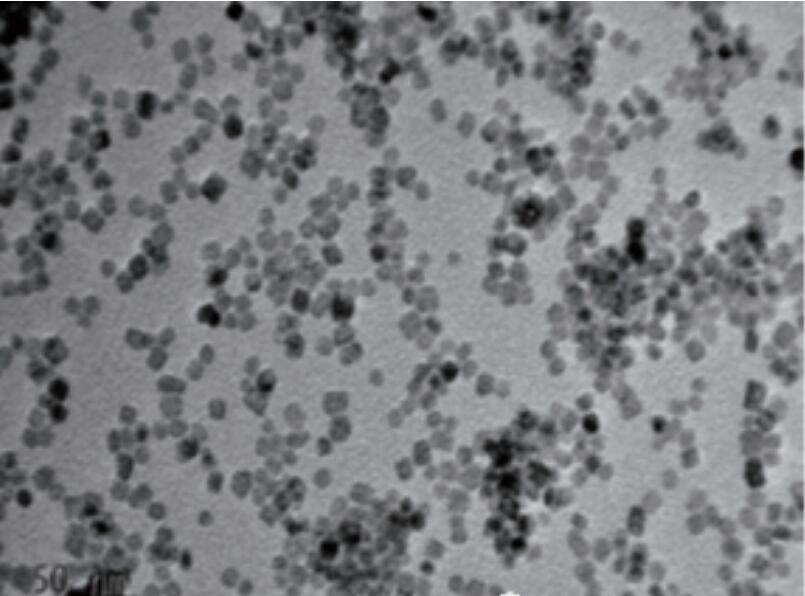
Wadannan hanyoyin guda biyu suna nuni zuwa ga hanyar shirya samfurori ta hanyar babban zazzabi da kuma hakkin kai tsaye a yanayin zafi a cikin rufaffiyar tsarin. Lokacin da sake yin amfani da ruwa ruwa ne, ana kiranta Hydrothermal hanya. A daidai da, lokacin da sauran rikitarwar da aka yi, ana kiranta hanya ce ta solvothermal. Abubuwan da Nano sun haɗa da barbashi na Nano suna da tsarkakakke, watsawa mai kyau, musamman powders na Nano tare da ƙirar ƙirar ƙira ko fuskoki daban-daban. Narke Cerium chloride a cikin ruwa distilled ruwa, saro kuma ƙara bayani sodium hydroxide. Haɗa hydrothermal a 170 ℃ na 12 hours don shirya Cerifide Nanorods tare da fallasa (111) da (110) crystal jirage. Ta hanyar daidaita yanayin dauki, rabo daga (110) jirage na (110) Crystal jirage a cikin filawar da aka fallasa Crystal jirage za a iya ƙaruwa, ci gaba da inganta ayyukan catalytic. Daidaita abubuwan da suka faru da kuma Ligands na samaniya na iya haifar da Nano Ceria tare da Hydrophility na Musamman ko Lipophilicity. Misali, ƙara oions ion ga mai ruwa mai ruwa zai iya shirya monodisperperperperperperasse hydrophaidmics ruwa Cerium oside kayan abinci na ruwa a cikin ruwa. Ta hanyar zabar wanda ba mai yawa ba kuma gabatar da Oleic acid a matsayin Lignd a lokacin da aka amsa, Mondisperperperperperperperse lipophilic Ceria nanoparticles za a iya shirya a cikin abubuwan da ba polar kwayoyin cuta. (Duba siffa 1)
Hoto 1 Nano Ceria da Rod-dimbin yawa Nano Ceria
1.4 Sol Gel hanya
Hanyar Sol Gel ita ce hanya wacce ke amfani da wasu ko da yawa a matsayin masu wucewa kamar hydraysis a cikin ruwa bayan tsufa, sannan kuma a ƙarshe bushe da kuma lasisin don shirya sililine powders. Wannan hanyar tana dacewa musamman don shiri sosai Nano Ceria Fitar da Nanomaterials Cerium, Cerium Titanium, Cerium Zane Orodes, wanda aka ba da rahoton a rahotanni da yawa.
1.5 Sauran hanyoyin
Baya ga hanyoyin da ke sama, akwai kuma hanyar micro site, hanyar lantarki, plasma flame Contramuson hanya, ion-musayar membrane hanyar da yawa da sauran hanyoyin. Wadannan hanyoyin suna da babban mahimmanci ga bincike da aikace-aikacen Nano Ceria.
Aikace-aikacen 2-Nanometer Cerium Oxide a cikin ruwa magani
Cerium shine mafi yawan abinci a tsakanin abubuwan duniya mai wuya, tare da ƙarancin farashi da aikace-aikace masu yawa. Nanometer Ceria da kuma abubuwan da aka kwace sun jawo hankali sosai a fagen magani saboda babban yankin surshinsu, aikin catalyttitic da kuma kyakkyawan tsarin catalyttitt da kuma kyakkyawan tsari.
2.1 Aikace-aikacen aikace-aikace naNano Cerium OxideA cikin aikin ruwa ta hanyar adsorption
A cikin 'yan shekarun nan, tare da ci gaban masana'antu kamar masana'antun lantarki, babban adadin sharar gida ya ƙunshi zubu da tsire-tsire masu nauyi da ions mai nauyi. Ko da a hanyar gano hankali, yana iya haifar da babbar matsala ga kwayoyin ruwa da kuma yanayin rayuwar mutane. Hanyoyin amfani da aka saba sun haɗa da oxidation, flotation, Opensiption, NanoORPLPETREPTERS, Yankuna, da sauransu a cikin su, ƙarancin farashi, ƙananan tsada, da kuma ingancin magani. Kayan kayan Nano suna da babban yanki na samaniya da babban aiki a matsayin adsorbents, kuma akwai wasu rahotanni da yawa akan tsarin ƙwararru na Nano zuwa Adsorb kuma suna cire ions mai cutarwa da kuma cire ciyawar da ke tattare da su.
Bincike ya nuna cewa Nano Ceria tana da damar iya karfin Adsorving don F - a cikin ruwa a ƙarƙashin raunin acidic. A cikin mafita tare da farawar farko game da F - na 100MG / L da PH = 5-6, 23MG / g, da cirewa na F - shine 85.6%. Bayan saukar da shi a kan polyackrylic acid resin ball (loda kaya: 0.25g / g), karfin cire f --MG / l of f - ruwa mai kyau; Lokacin aiki sau 120 da girma, sama da 90% na F - ana iya cire shi. Lokacin amfani da Adsorb phosphate da iodate, damar adsorction na iya isa sama da 100mg / g a karkashin ingantaccen jihar Adsorction. Za'a iya sake sake amfani da kayan da aka yi amfani da shi bayan sauki dayan alloli da magani na hutu, wanda ke da babban fa'idodin tattalin arziƙi.
Akwai bincike da yawa a kan adsorption da lura da sexic mai guba kamar arsenic, chromium, cadmium, da kuma jagorantar amfani da Nano Ceria da kayan kwalliyar sa. Mafi kyawun adsorption ph ya bambanta da ions mai nauyi na karfe tare da jihohin daban-daban. Misali, yanayin alkaline yanayin tare da tsaka-tsaki na tsaka-tsaki yana da mafi kyawun yanayin adsorption, inda ake samun damar a ƙarƙashin raunin acidic, inda zaɓar da ke cikin rauni a matsayin 110MG / g a ƙarƙashin yanayin duka. Gabaɗaya, ingantattun kira na Nano Ceria da kayan haɗin sa na iya cimma babban adsorption da haɓakar riguna daban-daban.
A gefe guda, Cerium Oxide Omanomaterials suma suna da ficewar ayyukan adsorbing a cikin cirewar kwayoyin halitta wanda ke daɗaɗawa yana da cikakkiyar ja da distan da ke ciki, musamman a cikin cire Konotal. karfin 942.7mg / g a cikin minti 60.
2.2 Aikace-aikacen Nano Ceria a cikin Tsarin Shiga ciki mai yawa
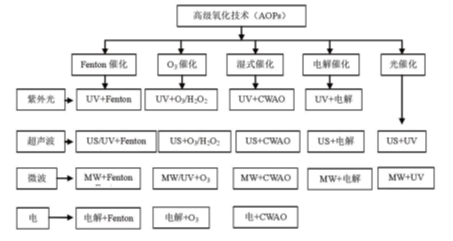
An samar da tsarin hauhawar abu mai amfani (kaya don gajeriyar hanya) don inganta tsarin jiyyar da ke da kullun. Tsarin tsari na ciki, wanda kuma aka sani da fasahar oxidation mai zurfi, ana nuna shi ta hanyar hydroxyl Hydroxyl rydroxyl (O2 -), Superletica tsattsauran iskar oxididgen. A ƙarƙashin yanayin hawan zazzabi da matsin lamba, sauti, iska mai sauƙi, ozon oxidthatation downcals mai ban tsoro, catalytic hade da na lantarki, da sauransu (duba adadi 2).
Hoto na 2 Classigfication da Haɗin Fasaha na Tsarin Haske mai tasowa
Nano Ceriashine mai haifar da maganin cuta na yau da kullun a cikin tsarin shafafunsu na sama. Saboda saurin juyawa tsakanin CE3 + da Ce4 + da saurin rage yawan iskar shaye-shaye da aka kawo da kuma sakewa, Nano Ceria tana da ikon catalytic. A lokacin da aka yi amfani da shi azaman mai gabatarwa, zai iya inganta ikon catalytic da kwanciyar hankali. Lokacin da ake amfani da Nano Ceria da kayan haɗin sa azaman masu conlysts, kaddarorin catalytic ya bambanta sosai tare da ilimin halittar cryaly, girman abubuwan da ke da manyan abubuwan da suka shafi aikinsu da aikace-aikacensu. An yi imani da cewa ƙananan barbashi da mafi girma shafin yanar gizo, da mafi yawan shafin karfin gwiwa. The catalytic ability of the exposed crystal surface, from strong to weak, is in the order of (100) crystal surface>(110) crystal surface>(111) crystal surface, and the corresponding stability is opposite.
Cerium Oxide kayan abu ne na semiconductic. A lokacin da Nanometer Cerium Oxoraya ne ke da ƙarfi tare da makamashi sama da baginar band, da varenceand convents m, kuma tursar da abin da ake zartar da dabi'un ya faru. Wannan hali zai inganta yawan canji na I3 + da Ce4 +, wanda ya haifar da ingantaccen ayyukan Photocatalytic na Nano Ceria. Photocatalysis na iya cimma lalata kwayoyin halitta ba tare da tsarin sashe ba, don haka aikace-aikacen sa shine mafi yawan fasahar Nano Ceria a cikin filin nan. A halin yanzu, babban abin da aka mayar da hankali shine a kan lalata lalata na Azo-Dyes, phenol, chlorobenzenze, da kuma sharar magani ta amfani da castystates tare da abubuwan da aka tsara daban-daban. Dangane da rahoton, a karkashin ingantaccen tsarin mai kara da yanayin tsarin abubuwa, karfin lalata na iya kaiwa fiye da kashi 80, da kuma cirewa da cire kwayar cuta na carbon (toc) na iya kai sama da 40%.
Nano Cerium Oxide Catalysis don lalata cututtukan ƙwayar halitta kamar ozone da hydrogen peroxide wani fasaha ne na kirista sosai. Kama da Photocatalysis, shi ma yana mai da hankali kan iyawar Nano Ceria tare da morphologies daban-daban ko kuma Crystal wurare masu hade da lalata gurɓataccen ƙwayar cuta. A cikin irin waɗannan halayen, masu kara kuzari na iya bugun da tsararraki mai yawa daga cikin ozone ko hydrogen peroxide, wanda ke hydrogen peroxide, wanda ya kaiwa gurbata abubuwa masu lalata na ciki da kuma cimma nasarar ikon lalata operadation. Sakamakon gabatarwar da suke ciki a cikin amsawar, ikon cire mahaɗan kwayoyin halitta yana inganta sosai. A mafi yawan halayen, mafi kyawun cirewar na ƙarshe na abu mai manufa na iya kaiwa ko kusanci 100%, kuma farashin cire zuwa ga Tect shima ya fi girma.
A cikin hanyar ingancin iskar shaka ta oxidcatratation, kadarorin kayan haɗin oxygen tare da ƙwararrun juyin juya halin oxygentalytic don kula da gurɓataccen ƙwayar oxidic don kula da gurɓataccen ƙwayar cuta. Kayan Katolika abu ne mai mahimmanci wanda ke tantance h2O2, da samar da H2OCAl suna yanke hukunci game da ingancin iskar shaka-isarwa. Nazarin Gyaran kayan electrode ta amfani da Nano Ceria ya sami hankalin da aka yi tartsasawa biyu da na duniya. Masu bincike galibi suna gabatar da Otani Cerium Oxide da kayan haɗawa ta hanyar hanyoyin sunadarai daban-daban don gyara ayyukan lantarki, kuma hakan ya inganta ayyukan lantarki, kuma hakan ya inganta ayyukan lantarki da ƙididdigar ƙarshe.
Microwave da duban dan tayi galibi suna da mahimmanci matakan taimako don samfuran catalytic da ke sama. A ɗaukar taimako na ultrasonic a matsayin misali, ta amfani da raƙuman ruwa mai laushi tare da mituvencies da yawa, miliyoyin ƙananan kumfa ne a cikin mafita mai tsabtatawa musamman wakili na musamman wanda aka tsara. Wadannan ƙananan kumfa, yayin matsawa da fadada, a koyaushe, a koyaushe kayan kumfa da sauri, sau da yawa suna inganta ingancin catalytic.
3 Kammalawa
Nano Ceria da kayan haɗinta na iya magance ions da kuma zubar da gurnani a cikin ruwa, kuma suna da mahimmancin aikace-aikace a cikin filayen magani na ruwa. Koyaya, yawancin bincike har yanzu suna cikin dakin gwaje-gwaje, kuma don cimma saurin aikace-aikacen ruwa a nan gaba, har yanzu matsaloli masu zuwa suna buƙatar magance gaggawa:
(1) daɗaɗa babban shirin farashin NanoCeo2Abubuwan da ke tushen kayan ya kasance mai mahimmanci a cikin mafi yawan aikace-aikacen su a cikin maganin ruwa, waɗanda har yanzu suna cikin matakin binciken binciken. Binciken ƙananan farashi, sauki shirye-shirye hanyoyin shirye-shiryen da zasu iya tsara tsarin ilimin halittar jiki da girman kayan tushen Nano Ceo2 har yanzu suna mai da hankali kan bincike.
(2) Saboda ƙaramin kayan tushen Nano Shugaba na kayan aiki na Nano, sake amfani da batutuwa bayan amfani da mahimman abubuwan da ke iyakance aikace-aikacen su. Haɗin shi da kayan saiti ko kayan magnetic zai zama mabuɗin shugabanci na bincike don shirye-shiryen kayan aikinta da fasaha maimaitawa.
(3) Ci gaban tsari na haɗin gwiwa tsakanin Fasahar Ruwa na Nano da Fasaha na gargajiya zai inganta aikace-aikacen fasahar Nano Shugaba a fagen maganin ruwa.
(4) Har yanzu akwai har yanzu iyakantaccen bincike kan guba na kayan Nano Shugaba, da Halin muhalli da halayyar muhalli a cikin tsarin maganin ruwa ba a ƙaddara ba tukuna. Ainihin tsarin magani na yau da kullun ya ƙunshi haɗuwa da ɗimbin gurɓasassi, kuma masu maye gurbi zasuyi hulɗa tare da juna, don haka canza halaye da kuma yiwuwar halaye na nanomaterials. Sabili da haka, akwai buƙatar gaggawa don aiwatar da ƙarin bincike akan bangarorin da suka shafi.
Lokaci: Mayu-22-2023