Ma waena o nā pipi momonaʻole, he nui nā mea mechaninical, kiʻekiʻe a me nāʻano haʻahaʻa o ka mana, he nui. Hoʻohana pinepineʻia nā mea āpau.microroporpoous i kaʻoihana, akā e pili pololei ia i ka hana o Alumina, ke ola a me ke koho o Catalyst. ʻO ka laʻana, i ke kaʻina o ka hanaʻana o ka kaʻa kaʻaʻana, mai nā mea hoʻohui i waihoʻia mai nā pakani aila o ka pata i catalyst. Hiki ke hoʻohanaʻia e hoʻoponopono i keʻano o keʻano o ka hana a Almuina e hana ma ka hana ma I.improve i kāna hana catalytic.
Ua loaʻa i ka hopena kūpono, a ua hoʻokōʻia nā metala ikaika ma hope o ka heluʻana ma hope o ke aniani kiʻekiʻe. Kui pū kekahi me ka hoʻololiʻana o keʻano,ʻaʻole he huiʻo Messpoous, a hiki i ka petorton ka moku'āinaʻo Greatika, aʻaʻole hiki ke hoʻokō ka lani Pono pinepine ka hoʻomaʻamaʻa hoʻololiʻana e hoʻomaikaʻi i ka hana catalytic, me nā hui metaroo i loko o ka skeleton.
ʻO ka hoʻonohonohoʻana o ka Electron kūikawā o nā mea i loaʻa i nā mea i loaʻa i nā mea waiwai, nā mea kūʻai aku a me nā mea hoʻonaninani a me nā mea hana. Hiki i nā huahana Mesopor Moder Messoporous e hoʻoponopono i nā waiwai (Alkali) Ma kēia pepa, e hoʻolauna ka weheʻana a me ka hanaʻana o ka hoʻokōʻana i ka hana matekani, ka paepae paʻaʻana a me ka papa haʻahaʻa.
1 Ma hope o Mahope
1.1ʻO ka hoʻomākaukauʻana o Aluna Carrier
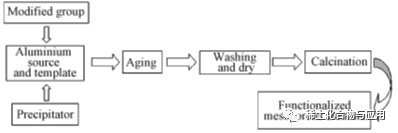
ʻO keʻano hoʻomākaukauʻana o ka lawenaʻo Anuina Carrier e hoʻoholo ana i kona hoʻokaʻawaleʻana i keʻano, a me kona mau mākaukau maʻamau e komo i keʻano a me nā mea kūʻai akuʻo Pseudo-BE ʻO Psedoboehmite (PB) i manaʻo muaʻia e calvet, a me ka pops i hāpaiʻia e loaʻa i ka mahana kiʻekiʻe Wahi a nā mea likeʻole i likeʻole, ua mahele pinepine ia i kaʻano o ka mana o ka crystalimanity, a ua hopenaʻia e ka hoʻonuiʻana i nāʻano hana.
Ua hoʻomākaukau pinepineʻiaʻo PB ma o keʻano hana. Hoʻohuiʻiaʻo Alkali i ka hoʻonāʻana a iʻole ka Acid i hoʻohuiʻia i ka hopena o ka Aluminate a loaʻa iā Alumina He maʻalahi keʻano o keʻano o ka hanaʻana a me ka haʻahaʻa ma ke kumu kūʻai,ʻo ia ka hoʻohana pinepineʻana i nā mea hana.). Ma keʻano kolepa, al (Oh) 3is i loaʻa e ka hopena o keʻano o CO2AT BO2, a hiki ke loaʻaʻo PB ma hope o ka wā. Loaʻa kēiaʻano hana i nā hana maʻalahi,ʻoi aku ka maikaʻi o ka huahana a me keʻano haʻahaʻa a me keʻano kiʻekiʻe ʻO ka alumini alkoxide ka hydrolyzded e hana i ka hana alumini oxide Eia naʻe, he paʻakikī ke kaʻina hana, a he mea paʻakikī ke ola hou ma muli o ka hoʻohanaʻana i kekahi o nā mea waiwai i loko o nā meaʻonaʻona.
Eia kekahi mea hoʻohuiʻia i nā paʻakai a me nā hoʻopiliʻana o nā metala I ke wā, ua hoʻomaikaʻiʻia ka neʻe o ka hoʻonuiʻana i ke kumu kūʻai nui, a ua pono keʻano o kānaʻoihanaʻoi aku ka maikaʻi o ka hanaʻana ma ke kula.
1.2 Ma ka hoʻomākaukauʻana
ʻAʻole hiki i ka hoʻohuiʻana o ke alaloa i nā koi hana, no laila he pono ia e hoʻomākaukau i ka hana kiʻekiʻe ma mua. ʻO nā ala synthesis e hoʻopili pinepine ai:ʻO keʻanoʻo Nano-Casting me ka carbon a me ka carbon Synthesis o SDA: Evaporate-incourat-indit selchuts
1.2.1 Eisapa hana
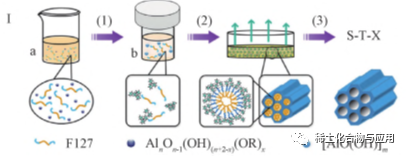
Hoʻohanaʻia kaʻano palupalu palupalu i keʻano acidic, e pale i ke kaʻina hana paʻakikī a hiki ke hoʻomaopopo i keʻano o ka membrane paʻakikī. Ua makemake nui ka hoʻomākaukauʻana o Maia ma o Eisa i ka nānā nuiʻana ma muli o kāna loaʻa maʻalahi a me ka hana hou. Hiki ke hoʻomākaukauʻia nāʻano hoʻololi likeʻole. ʻO ka nui o ka pore o ka mata e hiki ke hoʻoponoponoʻia e ka hoʻololiʻana i ka lōʻihi o ka mydrophobic F127, Triethanoxine (TrietHawaine ʻO ka hoʻomohalaʻana i ka meopophase i hoʻokumuʻia e nā micelles micelles ma Sol.
Ma ke kaʻina EISA, ka hoʻohanaʻana o nā mea hana non-aqueous (e like me ethanol) a me nā hui o ka genice Eia nō naʻe, ma loko o nā mea hana hoʻopunipuniʻole, nā mea hana a me nā mea hana e lilo ai nā mea kanu e nalowale i ko lākou hydrophinicity / hydrohobility. Eia kekahi, ma muli o ke kaliʻana o ka hydrolysis a me nā polyconderation,ʻo ka huahana hydrophobic, kahi mea paʻakikī e launa pū me nā mea e hoʻopili ai i nā mea. Wale nō i ka wā o ka nānāʻana o nā mea hana a me ke kiʻekiʻe o ka hydrolysis a me nā polycondentation o keʻano o ka hanaʻana i keʻano o keʻano o ke kaʻina No laila, he nui nā pakeneka nui e pili ana i nā kūlana o ka evaporation o nā hoʻoponopono a me ka lono o ka hydrokess, etc., e pili ana i ka pae o ka poʻokela hope loa. E like me ka hōʻikeʻia ma Fig. 1, o OMA Nā mea hana me ka nui o keʻano o ka hoʻokele kiʻekiʻe a me keʻano o ka hana catalytic e hoʻopiliʻia e ka Econoperage i kōkuaʻia Ua hoʻolahaʻia ka hoʻomaʻamaʻaʻana i ka hydrollus o ka hydrollys i nā papa hana a Aluminim e hana i nā huiʻo Allicasel Ma ka Hoʻolaha Eisational Easna, ua hele pūʻia ke kaʻina o ka lewa o ka hydroltication ma ka hydroltimanim of Orgealume, no laila he mana ka hopena o ka maʻi Oma. Ua hoʻolahaʻia ka hoʻomaʻamaʻaʻana i ka hana hoʻoponopono i ka hydrolysis piha o ka Alumini Hoʻohālikelikeʻia me ka Male ma keʻano kuʻuna,ʻo Oma ua hoʻomākaukauʻia e keʻano he nuiʻo Sa-Eisa, heʻoi aku ka maikaʻi. I ka wā e hiki mai ana, hiki ke hoʻohanaʻia ke ala esAa e hoʻomākaukau ai e hoʻomākaukau i ka apertra-nui ma keʻano me ka hoʻohanaʻoleʻana i nā mea hou.
Fig.
1.2.2 Ke kaʻina hana'ē aʻe
Hoʻololiʻo Mantna Hoʻolaha Ma Ke Kūleʻa Kūlohelohe o Synthesiter he Nā mea hoʻomohalaʻo synthesis e loaʻa i nā mea aʻoaʻo mestilasy ke hoʻololiʻia i kēia kaʻina mepaʻi. I kēia manawa, ua hōʻikeʻia nā mea aʻo he nui i ka synthesis o ka ma me nāʻano likeʻole. I nā makahiki i hala iho nei, e pili ana ka noiʻi ma luna o ka synthesis o Ma me ka Glucose, Super Loaʻaʻo Ctab i loaʻa i ka hoʻololi pololeiʻana o ka PB e like me ke kumu Aluminum. Ma me nā mea waiwai'ē aʻe,ʻo ia Al2o3) -1, al2o3) -2 a me Al2o3a ʻAʻole hoʻololi ka hoʻohuiʻana o nā mea hana i keʻano o ka crystant crystal interstal o ka pb, akā hoʻololi i keʻano o keʻano o nā'āpana. Eia kekahi,ʻo ka hoʻokumuʻana o Al2O3-3 i hoʻokumuʻia e ka adhesion o Nanooparticles State a me ka hoʻohuiʻana i ka Peg. Eia nō naʻe,ʻo ka nui o ka pākeke pore Eia kekahi, ua hoʻomākaukauʻia nā palladitium-nā catalyss e pili ana i ka synthetic ma keʻano he cartirat i kākoʻoʻia e al2o3-3
No ka manawa mua, ua hoʻomākaukauʻia me ka hapa nui o ka piapapa nui ma ka hoʻohanaʻana ma ka hoʻohana kalaʻana ma ka hoʻohanaʻana a me ka nui alumina-alumina-alumina-alumina-alumanimʻeleʻele blag abd. Ke kaʻina hana hana e pili ana i ke kaʻina hana ma ka haʻahaʻa haʻahaʻa a me ke koʻikoʻi maʻamau. ʻO nā'āpana paʻa i waihoʻia ma ke kaʻina hana e hoʻokaʻawale i keʻano o ke kaiāulu, a hiki ke hoʻopaʻaʻia me ka haʻahaʻa a iʻole e hoʻopiliʻia ma keʻano o ka fuler a iʻole ka hoʻohuiʻana i keʻano o ka filler. ʻO ka wahi kiko'ī o ka synthesized ma ka 123 ~ 162m2 / g,ʻo ka nui o ka nui ʻO ka waiwaiʻo Nano-sigized aʻo ka nui o ke crystal e pili ana i ka 11nm. ʻO SocID-State Synthesis kahi kaʻina hou e synthesize ma: hiki ke lilo e hoʻohana ai e hana i nā radiochemical abserte. Ua hoʻopiliʻia nā chryside, a me ka ambulate a me nā gluciume a me nā glucose raw i nā mea hou o ka miki (1.7TBQ / ML), no laila ua maopopo ka hoʻohanaʻana i ka hoʻohana nuiʻana o ka Dose131I [Nai] Calar Cancer.
E hōʻuluʻulu i, i ka wā e hiki mai ana, hiki ke hoʻomohalaʻia nāʻano moloka Eʻimi i nā'ōkuhi pale a me nā kumuwaiwai aluminim, e hoʻopiʻi i ke kaʻina synthesis, e hoʻomaʻemaʻe i ka mechanis s synthesis a alakaʻi i ke kaʻina hana.
Ka hoʻololi hoʻololi o 2 ma
ʻO nāʻano hana o ka hoʻokaʻawaleʻana i nā'āpana mana ikaika ma ka mālamaʻana, me ka hoʻololiʻana, me ka hoʻoponoʻana,ʻo Kono mua,ʻo ia ka mea mua.
2.1 I loko o keʻano synthes synthesis
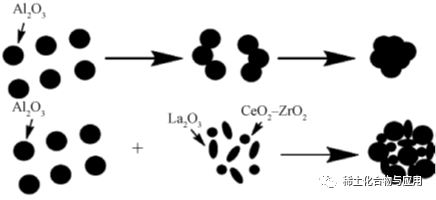
Hoʻohuiʻia nā hui i ka hoʻololi hana hana ma ke kaʻina hana o ka hoʻomākaukauʻana ma MA E hoʻololi a hoʻoikaika i ka hana skeleton a hoʻomaikaʻi i ka hana catalytotic. Hōʻikeʻia ke kaʻina hana ma ke kiʻi 2. Liu et al. syntheszed ni / mo-al2o3in a me p123 e like me ka hoʻohālikelike. Ua hoʻopiʻiʻiaʻo Ni lāuaʻo Ni lāuaʻo Mīpī i Serosents, me ka lukuʻana i ka hana a me ka hana limuni o keʻano. ʻO ka hāpaiʻana i kahiʻano ulu ulu lāʻau ma luna o kahi gamma-al2o3substorate, hoʻohālikelikeʻia me ka nui o ka heluʻana me ka nui o ka nui o ka helu. Mno2-Al2o3Na Nā Reping Hōʻike Hoʻohui a me ka Pono kiʻekiʻe e pono ai no F-, a loaʻa ka nui o ka inoaʻoihana PH. ʻO ka hana houʻana o Mno2-Al2O3isʻoi aku ka maikaʻi ma mua o ka pono o γ-al2o.structural ikaika e hoʻonui houʻia. E hōʻuluʻulu i nā mea i loaʻaʻia e nā mea i hoʻololiʻia e ka synthesis maikaʻi loa, he mea maikaʻi loa i keʻano o ka huiʻana, a me ka hana
Fig.
2.2 ke kaʻina hoʻohālikelike
Ua hoʻolimalimaʻia nā mea i hoʻomākaukauʻia Cai et al. hoʻomākaukauʻia ma mai mai ka p123 e ka solution solution ke kaʻina hana, a hoʻopiliʻia ma kaʻenehanaʻo ASanol ma luna o ka hana AdSorple. Eia kekahi, belkacemi et al. Hoʻokomoʻia ma ZNCL2SOLDSOLSON ma ke kaʻina hana e loaʻa i nā mea i hoʻopaʻaʻiaʻo Zinc i kohoʻia a me ka nui o ka papa'āina. Hoʻohālikelikeʻia me ka hana synthesis i loko o keʻano o ka synthesis, he hana maikaʻi loa keʻano o keʻano a me ka ikaika o nā mea hana
3 holomua holomua
ʻO ka synthesis o ka rare life ma Me nā waiwai kūikawāʻo ia ka hoʻomohalaʻana i ka hoʻomohalaʻana i ka wā e hiki mai ana. I kēia manawa, nui nāʻano kuʻina o Surnthess. Hoʻokomoʻia nā'āpana hana i ka hana o Ma. ʻO ka wahi kiko'ī e pili ana i nā wahi kiko'ī, ka nui o ka nui a me ka'āpana o ka vitheter o Marmeter e hoʻoponoponoʻia e keʻano o keʻano a me nā aluminum. ʻO ka mahana o ka heluʻana a me ka manika tmmplate e pili ana i ka piliʻana i nā wahi ākea a me ka nui o ka nui o ka ma. Ua loaʻa iā Suzuki lāuaʻo Yuikuchiki e hoʻonuiʻia ka nui o ka heluʻana mai 500 ℃ a hiki i 900 ℃. Eia kekahi,ʻoi, ka hoʻomaikaʻiʻana i ka hoʻomaʻemaʻe māmā e hoʻohuiʻia i ka hana, nā wahi ākea, a me ka lehulehu o ka hana i ka Facility.
3.1 Haaheo AdsORotionation Adsorbennt
ʻO ka fluorine i ka inu wai inu ma china he meaʻino loa ia. Ma kahi o ka hoʻonuiʻana o ka hopena o ka fluorine zinc solfate solcount i ka nui o ka uila e pili ana i ka nui o ka uila. I kēia manawa,ʻo keʻano o ka adsorption ka mea maikaʻi loa i waena o nā hana maʻamau Hoʻoikaika i ka kalahalawai, ua hoʻohana nuiʻiaʻo Amorina, hoʻoikaikaʻiaʻo ALMENA a me kāna mauʻoi aku ka maikaʻi o Adsisation. Akā ua kaupalenaʻia e ka hui AdSorping maikaʻiʻole o ka fluoride, a ma ka ph <6 hiki ke loaʻa i ka hopena o ka honua. Kundu et al. hoʻomākaukau ma me ka nui o ka nui o ka fluorine fluorine aʻoi aku ka nui o 62.5 mg / g. ʻO ka hikiʻana o ka nui o ka maʻi maʻi o ka ma nāʻano he nui, e like me ka nui o nā hui a me ka hana o ka pākaukau.
Ma muli o ka nui o ka ACID ACID o LA a me ka ikaika paʻakikī o ka fluorine, aia he ikaika ikaika ma waena o La a me nā maʻi maʻi. I nā makahiki i hala iho nei, ua loaʻa kekahi mau aʻo kekahi aʻoʻo iaʻo LA me he modicer hiki ke hoʻomaikaʻi aku i ka huiʻana o ka maʻi. Eia nō naʻe, ma muli o ka paʻaha o ka lewa haʻahaʻa o nā haʻawina ma ka'ōpū,ʻoi aku ka nui o nā Honua i ka wai. Ma kaʻaoʻao'ē aʻe,ʻo ke kiʻekiʻe kiʻekiʻe o ka aluminam i loko o ka wai wai o ka wai i kekahi o nā pisons i ke olakino kanaka. No laila, pono ke hoʻomākaukau i kahiʻano o ka hoʻohuihui pōkole me ka paʻapiha aʻaʻohe ona hoʻokuʻu o nā kaʻina hana maʻi. Ua hoʻololiʻiaʻo Mad e ka la a me ce i hoʻomākaukauʻia e ke ala impregnation (La / Ma a me Ce / Ma). Ua hoʻoukaʻia nā'ōmole māmā o ka honua ma luna o ka manawa mua, e loaʻa ana i ka hana o ka maʻi hydrosenl hoʻomaikaʻi i ka hikiʻana o ka adsorping o ka fluorine, la / ma nā pūnaewele addroxyl addroxyl Me ka hoʻonuiʻana o kaʻike muaʻana,ʻo ka hikiʻana o ka adsorption o ka maʻi maʻi e hoʻonui ana i ka hopena o ka fluor. Eia kekahi, ua hikiʻole nā mea hoʻohālikelike o nā sulfate mau o nā kahe i ka Alumina i hiki i ka nui o ka nui o nā hiʻohiʻona. ʻOiaiʻo ka noiʻi pili e pili ana i ka piliʻana o ka'āinaʻo Alumina i laweʻia,ʻo ka hapa nui o ka noiʻiʻana i ka hana o ka flutorection SULFEE RAMPE ma Zinc HydromtateLurgy'ōnaehana, a hoʻokumu i kahi kumu hoʻohālike kaʻina no ka mālamaʻana i ka hopena o ke kino
3.2 Catalyst
3.2.1ʻO ka hana maloʻo maloʻo o ka methane
Hiki i ka mīkini ke kāpae i ka oihanaʻoi aku ka maʻi (mactity) o nā huahana kiʻiʻoniʻoni, hoʻonui i ka maʻi oxygen, a me ka hoʻopiliʻana i nā catalysts a me ka paʻaʻole. Hoʻohana pinepineʻia ia e kākoʻo i nā metala noble a me nā hoʻololiʻana i nā metala i ka catalyze i ka metation o ce2. I kēia manawa, e hoʻopiʻiʻia nā huahana mesoporous i nā mea hana maloʻo (MDR), Etc.) kumukūʻai no ka metane. Eia nō naʻe, ka hana lawehala a me ka paleʻana o ka carbon i ka pale o nā ni / al2o3lead i ka wikiwiki o ka catalyst. No laila, pono ia e hoʻohui i ka maʻi maʻi, hoʻololi i ka mea lawe moku hoʻokele a hoʻomaikaʻi aku i ka hana hoʻomākaukau e hoʻomaikaʻi ai i ka hana catalytic, Statibworstance. Ma ka laulā, hiki ke hoʻohanaʻia nā molila honua e like me nā mea hoʻolaha a me nā mea hoʻolaha uila ma Heterogeneous, a hoʻololi i nā waiwai o ke metallic
Ua hoʻohana nuiʻia e hoʻonui i ka pili o nā metala, a hāʻawi i ka pale no nā metala ikaika e pale ai i ko lākou agglomeration. Ua hoʻonuiʻo La2O3Wing kiʻekiʻe oxygen i ka pale o ka carbon i keʻano o ka carbon, a me ka la2o3promotes i nā hana a me ke kūleʻa houʻana. Hoʻonui ka La2O3PROMOTOTER i ka hana MDR o ka CO / MA Catalyst, a me COWET4 BIETORECT i ka papa liʻiliʻi o 8nm ~ 10nm ~ 10nm ~ 10nm ~ 10nm ~ 10nm ~ 10nm ~ 10nm ~ 10nm ~ 10nm ~ 10nm ~ 10nm ~ 10nm ~ 10nm ~ 10nm ~ 10nm ~ 10nm ~ 10nm ~ 10nm ~ 10nm ~ 10nm. Ma ke kaʻina hana MDR,ʻo ka hoʻopiliʻana ma waena o La2o3And C2omed La2o2co3Mesco3 La2o3promos Hydrogen i ka hāʻawiʻana i ka nui o ke koho balota kiʻekiʻe a hoʻonui i ka maʻi oxygen ma 10% c? ʻO ka hoʻohuiʻana o La2o3redures i ka ikehu e hana ana i ka ikehu o ch45. No laila, kohoʻia ke kaula o ch4increas i ka 93.7% i 1073K K.ʻO ka hōʻemi o ka hana ma La2o i hāʻawiʻia i ka hōʻai'ē
Ua kākoʻoʻiaʻo CE a me PR ma N / Al2O3calyst e like me ka like me ka loliʻana o ka volumegnation ma li xiaofeng. Ma hope o ka hoʻohuiʻana i ka sE a me PR,ʻo ke kahe i ka H2increased a me ke koho e emi ana. Ua hoʻololiʻiaʻo MDR e PR ua loaʻa i ka hopena catalytic maikaʻi loa, aʻo ka koho i H2increased mai 6,5.5% Ua hoʻohanaʻiaʻo Sol-Gel i keʻano, ua hoʻomākaukauʻiaʻo CE-Moded Malu me ka Alumini IsoproPoxide, SeriumPinil Servent a me C Cerum NATRAZATRATRAEL. Ua hoʻonui iki iki ka wahi o ka papa kiko'ī o ka huahana. ʻO ka hoʻohui o ka ce i hōʻemi i ka hoʻohuiʻana o ke koʻokoʻo e like me NaNoport ma ka papa. ʻO kekahi mau hui hydroxyl ma ka papa o γ- Al2o3wee i uhiʻia e nā cospounds ce compounds. Ua hoʻomaikaʻiʻia ka piʻiʻana o ka grommal, aʻaʻohe loli o ka crystal i hiki mai ma hope o ka hoʻohuliʻana i ka hoʻomakaʻana ma 1000 no 10 mau hola.wang Batowei et al. hoʻomākaukau ma nā mea ma ka waihona COO2-Al2O4by CopRecipitation CopRercipitation. Ua hoʻopiʻi pinepineʻia nā BUSO2? Ma hope o ke kākoʻoʻana i ka CO a me ka mo o CEO2-Al2O4
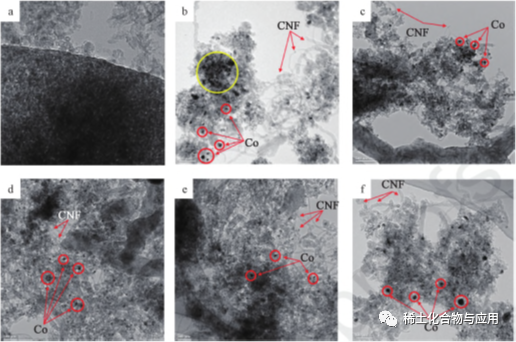
ʻO nā mea hoʻolaha e pili ana i ka Honua 3. Hiki i nā mea hoʻolaha ka Honua ke hoʻomaikaʻi i ka mea hoʻopiʻi o ka CO i ka carpers a me ka inhibit i ka agglometion o nā'āpana. ʻO ka liʻiliʻi o ka'āpana liʻiliʻi, ka mea ikaika i ka co-MATERY, ka ikaika o ka catalytic a me ka lawehala o kekahi mau hoʻolaha ma ka hana MDR i ka hana MDR i ka hana MDR i ka hana MDR. 4 he kiʻi hrem ma hope o ka mālamaʻana ma hope o ka mālama mdr ma 1023K, CO2: Ch4: N2 = 1: 1: 3.1 no 8 mau hola. Aia i nā'āpana'āpana ma keʻano o nā wahiʻeleʻele,ʻoiai nā mea lawe i keʻano o ka hina hina, e hilinaʻi ana i kaʻokoʻa o ka electron. Ma ke kiʻi HRTE me 10% CO / MA (PERS. 4B) Uaʻoi aku ka maikaʻi o Yco / Ma ma ke kamaʻilio ikaikaʻana, aʻo kāna hana hewa,ʻoi aku ka maikaʻi ma mua o nā mea hana'ē aʻe. Eia kekahi, e like me ka hōʻikeʻia ma nā kiʻi. 4B a 4F, HULLS Calbon Nanowires
FIG. 3ʻO ka hopena o ka hui pūʻana o ka honua ma keʻano o ke olakino a me nā mea kūʻai aku a me ka hana mdr catalytic o co / ma catalyst
3.2.2 Chekolidal
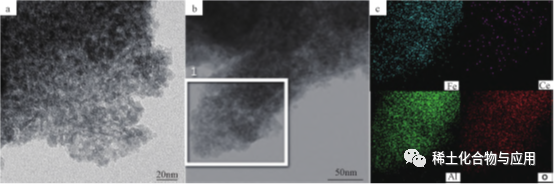
FE2O3 / memo-see, he ce-dored fet deoxidation cealyst, ua mākaukau e ka syntdronintant Ua hoʻokaʻawaleʻia nā ce i ka Alumina Matrix, a me F2O3 / Meso i kahi o nā mea kūʻai aku a me ka maikaʻi o ka CO2. E like me ka hōʻikeʻana ma ke kiʻi 5, e hōʻike ana nā kiʻi kiʻi ma Fe2O3 / memo-ceal-like i ka hana hewa i ka hana a me keʻano he channel. ʻO ka huiʻana o nā mea waiwaiʻo Geal i ka hālāwaiʻana o ka mea kūʻai aku i keʻano o ka neʻeʻana o nā kaʻa kaʻa, e ulu ana ka palekana o ka mīkini.
3.2.3 Catalyst no nā kaʻa
Ua kākoʻoʻo PD-RH i keʻano he nui a Alminus Hiki i ka hui pūʻo Aluminous e pili ana i ka Honua PD-RH / Alc / Alc Hoʻopili i kahi ala hana hydopormal hoʻokahi e hoʻomākaukau ai i kēlā me kēia hui pū me keʻano o ka huiʻana o nā mea hoʻonaninani o ka pūnaewele.
Fig. 4 mau kiʻi HRTEM o MA (A), CO / MA (B), Laco / Ma (e)
Fig. 5 Tam Image (A) a me EDS EDS koho (B, C) o FE2O3 / memo-ceal-100
3.3 hana maikaʻi
E maʻalahi maʻalahi nā mea uila o nā mea uila e like me ka hoʻololiʻana ma waena o nā pae o ka ikehu a me nā kukui māmā. Hoʻohana pinepine pinepineʻia nā'ōiwi honua e like me nā mea hana e hoʻomākaukau ai e hoʻomākaukau i nā mea likeʻole. Hiki ke hoʻoukaʻia nā'ōmole o nā nuas o nā kaha o Aluminum phosphate hollow a me nā mea hoʻololi: ʻO ka lōʻihi o ka lōʻihi o ka lōʻihi o ka lōʻihi ma kaʻaoʻao o Ultraviiole ʻO kēia mau mea i hoʻopaʻaʻia me nā kiʻiʻoniʻoni me ka lōʻihi o kaʻaoʻao optical ʻO ka loaʻaʻana o ka loaʻaʻana o nā mea waiwai me nā mea likeʻole i hoʻonuiʻia i nā waiwai o nā mea kanu, kahi e hiki ai ke hoʻolālā i nā sensor. ʻO ka hoʻomakaʻana o nā kiʻiʻoniʻoni o Mayyydroxide i ka hoʻolālāʻana o nā polokalamu optical e hōʻike ana no ka mea he like ka mea i kohoʻia.
3.4ʻO ka hoʻomauʻana
Me ka hoʻonuiʻana o ka mahana, ka mea e hoʻopilikia koʻikoʻi i ka hopena o ka catalyst Loaʻa i nā huahana Rareport i loaʻa i nā mea olakino maikaʻi a me ke kūpaʻa a me ke kūpaʻaʻana, kiʻekiʻe, kiʻekiʻe a maʻalahi a maʻalahi hoʻi ʻO ka hoʻohuiʻana o nāʻanuʻu o Rare Kīkī Hiki ke hoʻomaikaʻi i ka hoʻouluʻana i ka maʻi lapaʻau, a me nāʻano'āpana o ka mea lawe mai a me nā koina o ka laweʻana. ʻO LU UPUGUANG a me nā poʻe'ē aʻe i loaʻa e pili ana i ka hoʻohuiʻana o nā'āina nui, a iʻole ka hoʻololiʻana i nā meaʻala He kiʻekiʻe a me ka nui o ka Alumina i loaʻa i kahi ākea kiʻekiʻe a me ka nui o ka nui o ka nui.however Li yanquu et al. Hoʻohuiʻia 5% la2o3to γ-Al2O3, e hoʻomaikaʻi i ka palena o ka lewa a hoʻonui i kahi ākea o Alumina Carrier a me ka papa kuhikuhi E like me kaʻikeʻia mai ke kiʻi 6, La2o3added i γ-Al2O3, hoʻomaikaʻi i ka paʻa o ka mea lawe i ka wai i ka laweʻana i ka mea lawe i ka wai.
I ke kaʻina o ka hanaʻana i nā'āpana Nano-fibous me La i Mare,ʻo ka pae kiʻekiʻe o Ma-la iʻoi aku ka nui o ka mahana o Ma-lake me ka nui o ka nui o ka mahana ma ka hana nui. E like me ka hōʻikeʻia ma Fig. 7, me ka eleli ana, LA Maki i ka hopena o ka palaoa a me ke loliʻana,ʻoiai nā fits. ʻO 7a a me 7C Hōʻike i ka hōʻiliʻili o nā'āpana o Nano-Fibous. i Fig. 7b, keʻano o ka'āpana nui o nā'āpana nui i hanaʻia e ka heluʻana ma 1200 ℃ e pili ana i 100nm. Eia kekahi, hoʻohālikelikeʻia me MA-1200, MA-LA-1200ʻaʻole i hoʻohālikelikeʻia ma hope o ka mālama wela. Me ka hoʻohuiʻana o LA,ʻo Nano-Fiber i loaʻa ka maikaʻi o ka mea hiki. ʻOiai ma ke kiʻekiʻe kiʻekiʻe o ka heluʻana,ʻo Docopen i kahi i hoʻokaʻawaleʻia ma ka papa. Hiki ke hoʻohanaʻiaʻo LA i ka mea i hiki ke hoʻohanaʻia e like me ka laweʻana o ka mea lawe i ka PD Catalyst i C3H8OXIdation.
Fig. 6 papa hana hoʻolālā o ka lawehala alum a me kaʻole o nā meaʻole o ka honua
Fig. 7 mau kiʻi kiʻi o Ma-400 (A), MA-1200 (B), MA-La-1200 (D)
4 Ua hopena
ʻO ka holomua o ka hoʻomākaukauʻana a me ka hana hana hana o ka honua o ka Honua Ua hoʻohana nuiʻiaʻo Rare Honua Maʻa. ʻOiai ua hana nuiʻia ka noiʻiʻana ma ka noi catalytic, ka paʻaʻana o ka mea a me ka adsorption, he nui ka nui o nā kumukūʻai Eia ka hana e hiki ai i ka hana e hiki mai i ka wā e hiki mai ana: e hoʻonui i ke kikowaena me ka papaʻana o ka honua holoʻokoʻa: E koho i ke kaʻina hana kūpono; Hoʻokumu i kahi kaʻina hana kaʻina hana e pili ana i ke kaʻina hana e hōʻemi ai i nā kumukūʻai a hoʻomaopopo i ka hanaʻenehana; I mea e hoʻonui ai i nā pono o nā kumuwaiwai o Kina, eʻimi mākou i ka hoʻonohonohoʻana o ka manaolana o Raroth Rab Honua.
Pūnaewele Pūnaewele: Sanaxiʻepekema a me kaʻenehana Officeant offrall ofnont Profile (2011kdz01-04-01); Shaanxi stovince 2019 Science Science Science Project (19JK04490); 2020 Hoʻohālike Kūʻai Kūʻai Kūʻai Kūʻaiʻo Huaqing, xi 'he kulanui o ka pūnaewele a me kaʻenehana (20ky02)
Source: rare honua
Post Time: Jun-15-2021