Ceo2he mea koʻikoʻi nui o nā waiwai honua. 'Ōleloka Mokupuni Honua KipukaLoaʻa i kahi hana uila uila'ē aʻe - 4f15d16s2. Hiki i kāna mea kūʻai aku 4f kūikawā e hiki ke mālama pono a hoʻokuʻu i nā electrons, e hana ana i nā cerium i ka'āinaʻo + 3 valence state a me + 4. No laila, ua loaʻa nā huahana Ceo2 i nā lua oxygen i nā maʻi oxygen, a loaʻa i ka hiki ke kūʻai a hoʻokuʻu i ka oxygen. ʻO ka hoʻololiʻana o ka ce (iii) a me ce (iv) e hoʻopau pū i nā huahana ceo2 me nā mea i hoʻopiliʻia. Ua hoʻohālikelikeʻia i nā mea waiwai,ʻo Nano ceo2, e like me keʻano o ka mea i loko o ka wahi o ka oxygen. Aia he nui o kēia mau noiʻi noiʻi a me nā noi pili e pili ana i ka poʻe ceo2 e like me nā mea lawe stalyst, a me nā mea hana ikaika, a me nā hoʻolaha.
1.ʻO keʻano hoʻomākaukauʻana o ka nnometerKaniʻo Turide
I kēia manawa, nā hana hoʻomākaukau maʻamau no ka nano ceria nui loa i keʻano o keʻano a me keʻano kino. Wahi a nāʻano hana likeʻole, hiki ke kauʻia nā kumu lāʻau i keʻano o ka hōʻoia, hyderommal key ʻO keʻano kino ka mea nui loa keʻano o keʻano.
1.1ʻO ke ala Grinding
ʻO ke ala o keʻano o ka hoʻomākaukauʻana iā Nano Ceria maʻamau e hoʻohana nui ana i keʻano o ka one ʻO kēia ke ala e hoʻomaʻamaʻa nui ana i kaʻoihanaʻo Nano Ceria. ʻO ka laʻana, ka hoʻomākaukauʻana o Nano Cerumumʻo PWETS ADOPTS a iʻole ka mālamaʻana i nā mea kūʻai aku o ka cerium. Ma ka hoʻohanaʻana i nā'āpana likeʻole i ka nui o nā bear bean,ʻo Nano ceria me D50 mai nā haneli he mau haneli mau haneli mau haneli mau makahiki i hiki ke loaʻa ma o ka hoʻoponoponoʻana.
1.2 HōʻailonaʻOihana Makana
E pili ana keʻano o keʻano o keʻano o ka hoʻomākaukauʻana i ka pauʻana i ka pauʻana, e hoʻokaʻawale, holoiʻole, a me ka hoʻopauʻana o nā mea waiwai i nā wahi kūpono. Ua hoʻohana nuiʻia keʻanoʻana o ka manaʻo.ʻO nā kumu kūʻai nui, me nā kumu mua e like me ke hoʻomākaukauʻana, kiʻekiʻe. He ala maʻamau ia e hoʻohana ai no ka hoʻomākaukauʻana iā Nano Ceria a me kāna mau mea hoʻohui i nāʻoihana. Hiki i kēiaʻano ke hoʻomākaukau i ka kahuʻoleʻo Nano cerhialogy me ka mana o ka hoʻololiʻana, e hoʻopiʻi ana i nāʻano hana kolohe, a me ka ukuʻana o Nano ceralposition ma ka manaʻo mua. ʻO nā mea'ē aʻe,ʻo Cerum Itions hiki ke hoʻopauʻia e OH
1.3 Hydrothermal a me nā hana hoʻonā
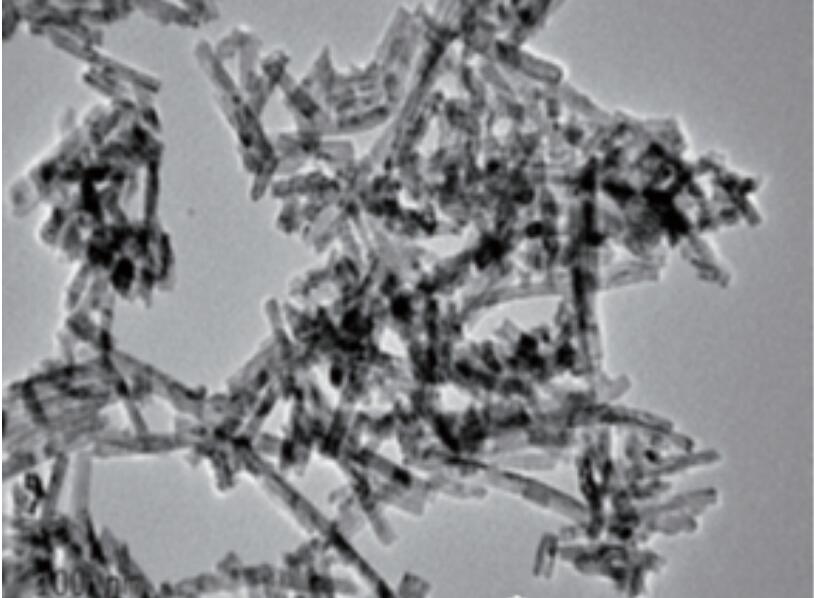
Hōʻike kēia mau hanaʻelua i keʻano o ka hoʻomākaukauʻana i nā huahana e nā kiʻekiʻe kiʻekiʻe a kiʻekiʻe a me keʻano kiʻekiʻe ma keʻano koʻikoʻi. I ka wai e hoʻopau ai ka hopena o ka hopena, ua kapaʻia ia i keʻano hydrotermal. ʻO keʻano, i ka wā e pau ai ka hopena o ka hopena i ka solivent. ʻO nā mea nui o nā mea nui e loaʻa ana i nā lāʻau lapaʻau nui, maikaʻiʻole a me nā mea'ē aʻe,ʻoi aku ka nui o nā powders nano a iʻole he mau morphologies o nā morphologies likeʻole. Hoʻopili i ka Csium Crium Serium React hydrothermal i 170 ℃ no 12 mau hola e hoʻomākaukau ai i nā mokuleleʻo Cerium ma Exposed (111) a me (110) a me (110) Ma ka hoʻoponoponoʻana i nā kūlana kūpono, keʻano o nā mokulele crystal (110) i nā papa crystal ma nā papa crystal i hiki ke hoʻonuiʻia, e hoʻonui hou i kā lākou hana catalytic. Hoʻoponopono i ka hopena o ka hopena a me nā ligands o nā ligands e hiki ke hana hou i nā'āpana o Nano Ceria i nā'āpana kūikawā me ka hydrophinity kūikawā a iʻole lipophilicity. No ka laʻana, e hoʻohui i nā Actete ISATE IS i ka PHESIRICE ELS MOODISHERSERSE ORDER OFDROPILIC TERDROPILIC i ka wai. Ma ke kohoʻana i kahi leka uilaʻole a me ka hoʻolahaʻana i ka OLECT ACID ma keʻano he lipophilic ceria nanoporicle (E nānā i ke kiʻi 1)
Kiʻi 1 Monodispersep
1.4 Sol Hol Gel Rworth
ʻO ke ala Gel Gel Gel kahi ala e hoʻohana ai i kekahi a iʻole kekahi mau hui e like me ka nui o nā mea kanu e like me ka hydrolysis ma hope o ka hanaʻana o ka wai. Ua kūpono kēiaʻano hana no ka hoʻomākaukauʻana i nā palu nui e likeʻole, e like me ke kolamuʻo CerIlate, Cerum nui,ʻo Cerum
1.5 mau ala'ē aʻe
I kākāʻia ma nā papa hana, aia hoʻi he mele aloha,ʻo Microrove synthesis ala hana,'Āpana Pāʻani BESMós. He mea nui kēia mauʻano no ka noiʻi a me ka noi o ka nano ceria.
ʻO ka noiʻana o 2-nanometer cerium oxde ma ka mālama wai
ʻO Cerumum ka mea nui loa i waena o nā mea i waena o nā wahi ākea, me nā kumukūʻai haʻahaʻa a me nā noi nui. ʻO Nanomeer Ceria a me kāna mau composites i hoihoi nui i ka mālamaʻana i ka mālamaʻana i ka wai ma muli o ko lākou wahi kiʻekiʻe a me nā hana maikaʻi loa.
2.1 palapala noiNano Cerium'OlumiI ka mālamaʻana o ka wai ma keʻano o ka adsorption
I nā makahiki i hala iho nei, me ka hoʻomohalaʻana o nāʻoihana e like me nāʻenehana uila, he nui ka nui o nā mea hoʻohuiʻia e like me nā mea hoʻohui lāʻauʻole e like me nā mea nui a me nā mea momona. ʻOiai ma nāʻike trace, hiki iā ia ke hōʻeha nui i nā mea aquatic i nā mea ola a me keʻano o ke ola kanaka. Hoʻohana pinepineʻia nā hana maʻamau, fleatation, rever, naminos me ka heihei maʻalahi, a me nāʻoihana haʻahaʻa, a me nā haʻawina haʻahaʻa haʻahaʻa, a me ka hoʻomaikaʻiʻana. Loaʻaʻo Nano reo nā huahana Noan i kahi kūlana kiʻekiʻe a he nui ka hana ma nā haumāna e like me nā nūpepa me nā mikaaha
Ua hōʻikeʻo ka noiʻiʻanaʻo Nano Ceria i ka nui Adsorption ikaika no F - i ka wai ma lalo o nā kūlana acidic nāwaliwali nāwaliwali. I kahi hopena me kahi mea e pono ai keʻano o ka F -ʻo 100MG / L a me PH = he 9-6. Ma hope o ka hoʻoukaʻana iā ia ma kahi o ka pôpekuʻo Palyucrylic acid bood back (ka nui o ka nui: 0.25g / g) Ke hoʻoili nei i ka 120 mau manawa i ka nui,ʻoi aku ma mua o 90% o f - hiki ke laweʻia. I ka manawa i hoʻohana ai iā AdSorb Phosphate a me Iodor, hiki i ka pono Adsorption ke hiki i ka 100MG / G ma lalo o ke kūlana AdSorption kūpono. Hiki ke kau houʻia ka mea i hoʻohanaʻia ma hope o ka heleʻana ma hope o ka mālama maʻalahi a me ka olakino,ʻo nā pono waiwai kiʻekiʻe.
Nui nā haʻawina ma ka Adserption ma nā'ōnaehana kaumaha kaumaha e like me Arsenanic, Chropium, a alakaʻi i ka hoʻohanaʻana i ka mea kūʻai aku. ʻO ka helu hoʻolaha hoʻolaha hoʻolaha hoʻolaha hoʻolaha PH e like me nā mea kila koʻikoʻi me nāʻano mea likeʻole. ʻO ka laʻana,ʻo ke kūlana alkaline i loaʻa me keʻano o ka neutral bias i loaʻa i ke kūlana adsorping maikaʻi loa no ka (III) ʻO ka holoʻokoʻa,ʻo ka synthesized synthesized o nano ceria a me kāna mau mea hoʻohui e hiki ai ke hoʻokō i nā mea hoʻohui kiʻekiʻe e like me nā kumukūʻai nui loa.
Ma kaʻaoʻao'ē aʻe,ʻo Cerum e pili ana i nā hana e pili ana i nā hana acid, e like me ka weheʻana o nā mea kūʻai aku hiki iā 942.7mg / g i 60 mau minuke.
2.2 Ke noi aku neiʻo Nano Ceria i ke kaʻina oxidand
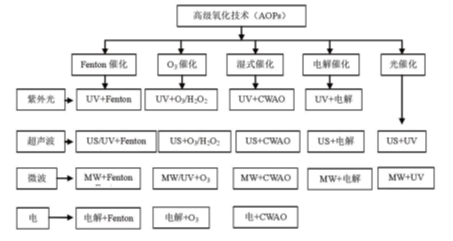
Hoʻolālāʻia ke kaʻina lāʻau lapaʻau (AOPS no ka pōkole) i manaʻoʻia e hoʻomaikaʻi i ka'ōnaehanaʻoihana hoʻomaʻamaʻaʻanoʻano hanohano. Hoʻonohonohoʻia e kaʻina hana hoʻomaʻamaʻa nui,ʻikeʻiaʻo ka Tech Technology hohonu,ʻo ia ka hōʻikeʻana e ka huaʻana o ka hyd = /), hoʻokahi oxiden, etoc. Ma ma o ke kūlana hoʻomaka o ka ea kiʻekiʻe o ke kiʻekiʻe a me ka huehue, kani, catakatika oxalication, e hoʻohui i ka hoʻohuihui hāmeʻa,
Kiʻi 2ʻO ka helu a me kaʻenehana hui pū o ke kaʻina Oxidation Advanced
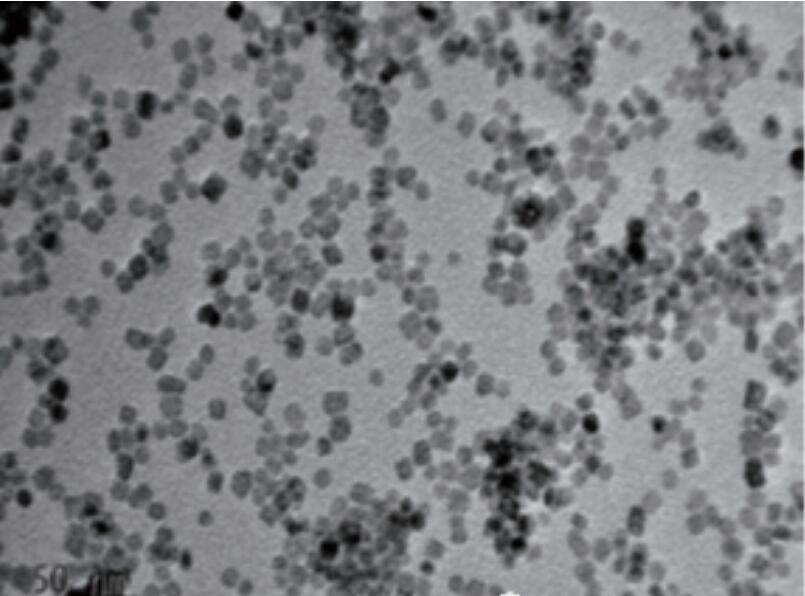
Nano ceriahe mea maʻamau i hoʻohana pinepineʻiaʻo Heterogeneous Ma muli o ka hoʻololi wikiwikiʻana ma waena o ce3 + a me ce4 + a me ka hopena rapid e laweʻia e ka oxygen i laweʻia e ka mana catalytic maikaʻi. I ka manawa i hoʻohanaʻia e like me kahi mea hoʻolaha catalyst, hiki iā ia ke hoʻomaikaʻi i ka hiki ke hoʻomaikaʻi i ka mana catalytic a me ka paʻa. Ke hoʻohanaʻiaʻo Nano Ceria a me kāna mau mea hoʻohui i nā catalysts, a me ka nui o nā meaʻona ʻO ka manaʻo maʻamau ua liʻiliʻi ka liʻiliʻi o nā'āpana a me ka nui o ka pae o ka papa, kaʻoi aku ka nui o ka pūnaewele, a me ka ikaika i ka Cattitalia. ʻO ka hiki i ka caterattic o kaʻaoʻao crystal i hōʻikeʻia, mai ka ikaika loa, aia ma keʻano o (1001) crystal modstal.
ʻO Cerumumx oxide kahi mea semiconductor semiconductor. Ke piʻiʻiaʻo Nanometer Cerium e nā kiʻiʻoniʻoni me ka ikaikaʻoi aku ka nui o kaʻoihana, a he mau mea kūʻai akuʻo Cancence. E hoʻolaha i kēiaʻano i ka huli hoʻololi o CE3 + a me CE4 +, e hopena ana i ka hana ikaika ikaika o Nano Ceria. Hiki i ke kiʻi kiʻi kiʻi ke hoʻokō i ka degradation pololei o ka hihia me kaʻole o ka hoʻopiʻiʻana, no lailaʻo kāna noiʻana i kaʻenehana nui o Nano Ceria ma Aops. I kēia manawa, aia ka manaʻo nui ma ka mālamaʻana i ka mālamaʻana i ka catalytic o Azoo, phermaceties Wahi a ka hōʻike, ma lalo o keʻano synthes catnthes i hoʻonuiʻia a me nāʻano hana hoʻohālikelike o kēia mau mea kūʻai aku ma mua o 80%.
ʻO Nano Ceriumx oversissi no ka debeadtation o nā mea waiwai o nā organic e like me Ozone a me Hydrogen Peroxide. Like me ke kiʻi kiʻi kiʻi, e pili ana i ka hiki iā Nano Cerhia I loko o nā hopena, hiki i nā catalysts ke hiki i ka hanauna nui o ka nui o nā mea ikaika loa mai ozone a iʻole ka hoʻokōʻana i nā mea hana hoʻonaninani maikaʻi. Ma muli o ka hoʻomakaʻana o nā mea i ka hopena i ka hopena,ʻo ka hiki ke wehe nui i nā mea hoʻokūkū hoʻokūkū. I ka hapa nui o nā hoʻohālikelike, hiki i ka helu wikiwiki o ka mea i hiki ke kau aku i ka waiwai i hiki ke kokoke a hiki ke hoʻokokoke aku i 100%, a kiʻekiʻe hoʻi ke kumukūʻai o ka hana.
I ka hoʻohuiʻana i ka hoʻohuiʻiaʻana i keʻano o ka minidation maʻamau, nā waiwai o ka'āpana o ka anidecon ke koho i ka koho balota E hoʻololi pono ka mea kālepa i ka hoʻoholoʻana o ka H2o2, a e kali ka hanaʻana i ka hoʻomaʻamaʻaʻana i ka maʻi uila me ka mālamaʻana i nā momona ma ka Humeikal. ʻO ke aʻoʻana o ka hoʻololiʻana o ka electrop Hoʻomaopopo ka poʻe noiʻi i nā mea noiʻiʻo Nano Ceruum a me kāna mau mea hoʻohui e hoʻololi ai i nā hana Electroxitika
ʻO Microwaved a me Ultrasound nā hana koʻikoʻi koʻikoʻi nui loa no nā hiʻohiʻona o nā catalytic ma luna. ʻO ka laweʻana i ke kōkua ultrasonic e like me keʻano he nui, e hoʻohana ana i nā nalu leo nui ma mua o 25khz i hanaʻia ma kahi o ka mea hoʻomaʻemaʻe maikaʻi loa. ʻO kēia mau pua liʻiliʻi, i ka wā o ka hoʻokūkū wikiwiki a me ka hoʻonuiʻana, e hana mau i nā mea hana braliat
3 huapono
Hiki ke mālama ponoʻiaʻo Nano Ceria a me kāna mau lako spospoite i nā mea kanu a me nā mea hoʻonaʻauao i ka wai, a he loaʻa nui ka noiʻana i ke kahua mālama wai. ʻAʻole naʻe,ʻo ka uku noiʻi nui hoʻi ma ke kahuaʻo ka hana ma ke kahua, a i hiki i ke hoʻokōʻana i ka noi Rapid ma ka wā e hiki mai ana.
(1) ke kumukūʻai kiʻekiʻe kiʻekiʻe o NanoCeo2Hoʻokomoʻia nā kumuwaiwai e pili ana i ka mea nui i ka hana nui o kā lākou noi i ka mālamaʻana i ka wai Keʻimi nei i nā uku haʻahaʻa haʻahaʻa, maʻalahi a maʻalahi hoʻi e hiki ke hoʻoponopono i ka morpology a me ka nui o na mea e pili ana i nā mea pili o Nano ceo ceo.
(2) Ma muli o ka nui o ka'āpana liʻiliʻi o Nano ceo ceo2 e pili ana i nā mea e pili ana i ka recycling a me nā mea hou aʻe e like me ka hoʻohanaʻana i nā mea hoʻohana nui i kā lākou noi. Ka huiʻana o ia me nā kumuwaiwai a iʻole nā mea hoʻonaninani e lilo i kumu noiʻi noiʻi no kāna mau mea kūʻai aku a me nāʻoihana hou.
(3)ʻO ke hoʻomohalaʻana o ke kaʻina hana ma waena o Nano ceo2 e pili ana i kaʻenehana lapaʻau waiʻaila wai no kaʻenehanaʻo Nano coayic i ka mahiʻai wai.
(4) ua palenaʻia ka noiʻi ma ka papa hano ceo ceo ceo ceo ceo e pili ana i nā'ōnaehana lapaʻau i ka waiʻole i hoʻoholoʻia ai ka wai no ka wai mālama waiʻole. E pili pinepine pinepineʻia ke kaʻina hana lapaʻau maoli i keʻano he nui o nā mea kōkua he nui, a me nā mea koho balota e launa pū ai me nā mea hoʻohālike o nā nanomateral. No laila, aia kekahi mea e pono ai e hoʻokō hou i ka noiʻi hou aku i nā hiʻohiʻona pili.
Post Time: Mei-22-2023