Uthi bewazi? Inqubo yabantu abatholayoytttriumyayigcwele ukusonta nezinselelo. Ngo-1787, iSusede Karl Axel Arrhenius yathola ngengozi i-Ore emnyama futhi esindayo emnyama egqemeni eliseduze kwedolobha lakubo laseYtterby Village futhi laliqamba ngokuthi "yterbite". Emva kwalokho, ososayensi abaningi kufaka phakathi uJohan Gadolin, u-Anders Gustav Ekberg, uFriedrich Wöhler nabanye baqhutshelwa ocwaningweni olujulile ngale ndo.
Ngo-1794, usomakhemikhali waseFinland uJohan Gadolin wahlukanisa ngempumelelo i-oxide entsha eYtterbium Ore wayiqamba ngokuthi yi-YTtrium. Kwakungokokuqala ukuthi abantu bathole kahle into evamile emhlabeni. Kodwa-ke, lokhu kutholwa akuzange kudonseke ngokushesha ukunakwa okusabalele.
Ngokuhamba kwesikhathi, ososayensi bathole ezinye izinto zomhlaba ezingandile. Ngo-1803, i-Klaproth yaseJalimane kanye neSweden Hitzinger noBerzelius bathola i-certium. Ngo-1839, amaSweden master atholakeleuhlobo linthanum. Ngo-1843, wathola i-Erbium futhii-terbium. Lokhu okutholakele kunikeze isisekelo esibalulekile socwaningo lwesayensi olandelayo.
Kwakungekho kuze kube sekupheleni kwekhulu le-19 lapho ososayensi bahlukanisa ngempumelelo into "yttrium" kusuka ku-yttrium ore. Ngo-1885, i-Austrian Wilsbach yathola iNeodymium ne-PraceDodymium. Ngo-1886, uBois-Baudran watholakaladysprosium. Lokhu okutholakele kuphinde kwacebisa umkhaya omkhulu wezinto zomhlaba ezingandile.
Ngaphezu kwekhulu leminyaka ngemuva kokutholwa kwe-YTTRIUM, ngenxa yokulinganiselwa kwezimo zobuchwepheshe, ososayensi behlulekile ukuhlanza le nto, futhi obuye babangela izingxabano namaphutha ezifundo. Kodwa-ke, lokhu akuzange kuyeke ososayensi emdlalweni wabo wokufunda i-YTTRIUM.
Ekuqaleni kwekhulu lama-20, ngentuthuko eqhubekayo yesayensi nezobuchwepheshe, ososayensi bagcina baqala ukukwazi ukuhlanza izinto zomhlaba ezingandile. Ngo-1901, iFrance u-Eugene de Marseille atholaindlu yokuhlala yokungenayogugu. Ngo-1907-1908, uWilsbach wase-Austrian noFrench u-Urbain walithola ngokuzimela i-lutetium. Lokhu okutholakele kunikeze isisekelo esibalulekile socwaningo lwesayensi olandelayo.
Kwisayensi yanamuhla nezobuchwepheshe, ukusetshenziswa kwe-yttrium kuya ngokuya kukhula kakhulu. Ngokuthuthuka okuqhubekayo kwesayensi nobuchwepheshe, ukuqonda kwethu nokusebenzisa kwethu i-YTtrium kuzoba nokujula okuningana.
Izinkambu zohlelo lokusebenza ze-ytttrium element
1.Ingilazi ye-Optical ne-Ceramics:I-YTtrium isetshenziswa kabanzi ekwakheni ingilazi ye-optical ne-ceramics, ikakhulukazi ekwenziweni kwama-ceramics asobala nengilazi ye-optical. Amakhompiyutha alo anezakhiwo ezinhle kakhulu zokukhanya futhi angasetshenziswa ukwenza izakhi zama-lasers, ukuxhumana kwe-fiber-optic neminye imishini.
2. Phosphors:Amakhompiyutha e-YTtrium adlala indima ebalulekile kuma-phosphors futhi angakhipha i-fluorescence ekhanyayo, ngakho-ke ajwayele ukusetshenziselwa ukukhiqiza izikrini ze-TV, abaqapheli kanye nemishini yokukhanyisa.Ytttrium oxideFuthi ezinye izinhlanganisela zivame ukusetshenziswa njengezinto zokwenziwa ze-luminescent ukuthuthukisa ukukhanya nokucaca kokukhanya.
3. I-Alloy Acditives: Ekukhiqizweni kwe-alloys yensimbi, i-yttrium ivame ukusetshenziswa njengesengezo sokuthuthukisa izakhiwo zemishini nokuphikiswa kokugqwala kwezinsimbi.Ytttrium alloyszivame ukusetshenziselwa ukwenza insimbi yamandla aphezulu futhiI-Aluminium Alloys, ukubenza bamelane nokulimaza ukushisa ngokwengeziwe.
4. Catalysts: Amakhompiyutha e-YTtrium adlala indima ebalulekile kwamanye ama-catalysts futhi angasheshisa izinga lokuphendula kwamakhemikhali. Zisetshenziselwa ukukhiqiza amadivaysi okuhlanza izimoto eziphethwe yimoto kanye nama-catalysts ezinqubo zokukhiqiza izimboni, kusiza ukunciphisa ukuphuma kwezinto eziyingozi.
I-5. Ubuchwepheshe bokucabanga kwezokwelapha: I-YTTRIUM isotopes isetshenziswa ebukhwini bemicabango yezokwelapha ukulungiselela amasotopes we-radioacticiactive, njengokulebula ama-radiopharmaceuticals kanye nokuhlonza ukucabanga kwezokwelapha zenuzi.
6. Ubuchwepheshe be-Laser:I-YTtrium Ion Lasers iyi-laser ejwayelekile eqinile yombuso esetshenziswe ocwaningweni oluhlukahlukene lwesayensi, umuthi we-laser kanye nezicelo zezimboni. Ukwenziwa kwalawa ma-lasers kudinga ukusetshenziswa kwamakhompiyutha athile we-yttrium njengezingenziIzakhi ze-.ytttriumFuthi amakhompiyutha abo adlala indima ebalulekile kubuchwepheshe besimanje nezobuchwepheshe kanye nasembonini, ehilela izinkambu eziningi ezifana ne-Optics, isayensi yezinto zokwakha, kanye nemithi, futhi zenze iminikelo emihle kwinqubekela phambili yentuthuko kanye nentuthuko yomphakathi wesintu.
Izakhiwo ezibonakalayo ze-ytttrium
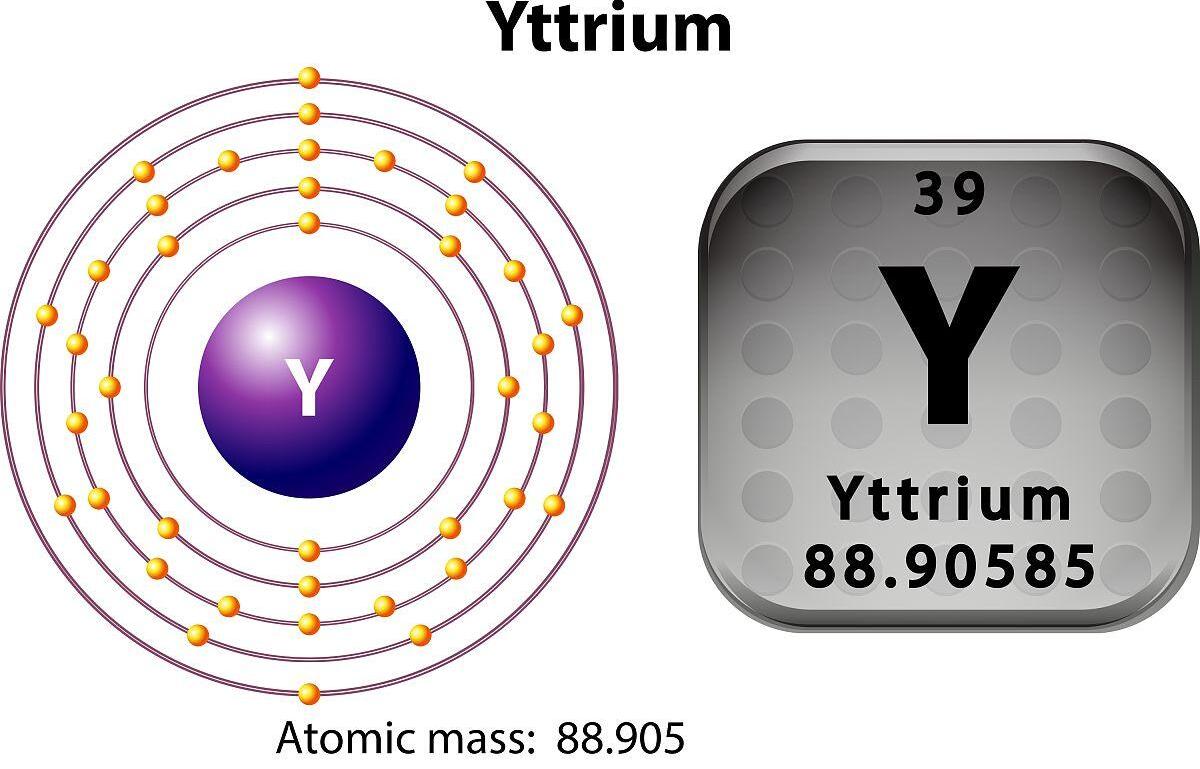
Inombolo ye-athomu yeytttriumuneminyaka engama-39 kanti uphawu lwamakhemikhali ngu-y.
1. Ukubukeka:I-YTtrium iyinsimbi emhlophe ye-silvery.
2. Ubuningi:Ubukhulu be-ytttrium ngu-4.47 g / cm3, okwenza kube yinye yezinto ezisindayo ekuqothulweni komhlaba.
3. Iphuzu elincibilikayo:Iphuzu lokuncibilika le-ytttrium lingama-1522 degrees Celsius (2782 degrees Fahrenheit), okusho amazinga okushisa lapho ushintsho lwe-yttrium lushintsha khona kusuka ku-ketshezi ngaphansi kwezimo ezishisayo ngaphansi kwezimo ezishisayo.
4 iphuzu elibilayo:Iphoyinti elibilayo le-ytttrium lingama-3336 degrees Celsius (6037 degrees Fahsius), okusho amazinga okushisa lapho ushintsho lwe-yttrium lushintsha khona kusuka ku-ketshezi ngaphansi kwezimo ezishisayo.
I-5. Isigaba:Emazingeni okushisa ekamelo, i-yttrium isesimweni esiqinile.
6. Ukuqhutshwa:I-YTTRIUM ingumqhubi omuhle kagesi onokuqhutshwa okuphezulu, ngakho-ke inezicelo ezithile ekukhiqizeni kwensiza ye-elekthronikhi nasekujikelezeni kwezesekethe.
7. Magnetism:I-YTtrium iyinto ebonakalayo ye-paramagnetic emazingeni okushisa asekamelweni, okusho ukuthi ayinakho ukuphendula kazibuthe okusobala kuma-magnetic amandla.
8. Isakhiwo seCrystal: I-yttrium ikhona ekwakhiweni kwe-hexagonal epakeke epakishwe.
9 IVolumu ye-Atomic:IVolumu ye-Atomic ye-YTTRIUM ngamasentimitha ayi-19,8 ngemvukuzane ngemvukuzane, ebhekisa kwivolumu elahlalwa yi-mole eyodwa yama-athomu e-yttrium.
I-YTTRIUM iyinto ye-metallic ene-density ephezulu kakhulu nephuzu lokuncibilika, futhi inokuqhutshwa okuhle, ngakho-ke inezicelo ezibalulekile ku-elekthronikhi, isayensi yezinto zokwakha kanye nezinye izinkambu. Ngasikhathi sinye, i-YTTRIUM nayo iyinto evamile engavamile, edlala indima ebalulekile kwabanye ubuchwepheshe obuthuthukile kanye nezicelo zezimboni.
Izakhiwo zamakhemikhali ze-ytttrium
1
2 Ku-Outer Electron ungqimba, i-yttrium inama-elektroni amabili we-valence.
I-3. I-Valence State: I-YTtrium ivame ukukhombisa isimo se-valence sika +3, okuyisimo esivame kakhulu, kepha futhi ingakhombisa izifundazwe zika-+2 no- +.
I-4. Ukuvuselelwa kwe-reaction: I-YTTRIUM iyinsimbi ezinzile, kepha kancane kancane izokwenza kancane lapho ivezwa umoya, yakha isendlalelo se-oxide ebusweni. Lokhu kubangela i-ytttrium ukuthi ilahlekelwe yi-luster yayo. Ukuvikela i-ytttrium, kuvame ukugcinwa endaweni eyomile.
5. Ukusabela nge-oxides: I-YTtrium iphendula nge-oxides ukwakha amakhompiyutha ahlukahlukene, kufaka phakathiytttrium oxide(Y2O3). I-YTtrium Oxide ivame ukusetshenziselwa ukwenza ama-phosphors nama-ceramics.
I-6.ytttrium chloride (Ycl3) nomaytttrium sulfate (Y2 (so4) 3).
7
8 9. I-Isotopes: I-YTTRIUM inama-isotopes amaningi, okuzinzile kakhulu okungukuthi yi-ytttrium-89 (^ 89y), enesigamu sempilo ende kanye ne-isotope. Ilebula.
I-YTTRIUM iyinto eqinile yensimbi enezindawo eziningi ze-valence kanye nekhono lokuphendula nezinye izinto ukwakha amakhompiyutha. Inezinhlobonhlobo zezicelo ku-Optics, isayensi yezinto zokwakha, umuthi, kanye nemboni, ikakhulukazi kuma-phosphors, ama-ceramic ezokukhiqiza, kanye nobuchwepheshe be-laser.
Izakhiwo zebhayoloji ze-ytttrium
Izakhiwo zebhayoloji zeytttriumezintweni eziphilayo zilinganiselwe.
1. Ukuba khona kanye nokufuna: yize i-YTtrium akuyona into ebalulekile empilweni, amanani e-Trace of yttrium angatholakala emvelweni, kufaka phakathi inhlabathi, amadwala, namanzi. Izinto eziphilayo zingangenisa amanani e-ytttrium nge-chain yokudla, imvamisa evela enhlabathini nasezitshalweni.
2 Amakhompiyutha amaningi we-YTtrium awamunwa kalula ezintweni eziphilayo, ngakho-ke zivame ukukhishwa.
3. Ukusatshalaliswa kwezinto eziphilayo: kanye esenhlekelele, i-YTTRIUM ihanjiswa kakhulu ezicubu ezifana nesibindi, izinso, amaSplen, amaphaphu, namathambo. Ikakhulu, amathambo aqukethe ukugxila okuphezulu kwe-ytttrium.
4. I-Metabolism kanye ne-Excretion: I-metabolism ye-YTTrium emzimbeni womuntu ilinganiselwe ngoba imvamisa ishiya umzimba ngokuxolelwa. Iningi lazo likhishwa ngomchamo, futhi futhi lingahle likhishwe ngendlela yokudukela.
I-5. Ubuthi: Ngenxa ye-bioavailability yalo ephansi, i-yttrium ayivamisile ukuqongelela amazinga ayingozi ezintweni ezijwayelekile. Kodwa-ke, ukuvezwa kwe-ytttrium ephezulu kungaba nemiphumela elimazayo ezintweni eziphilayo, okuholela emiphumeleni enobuthi. Lesi simo sivame ukwenzeka okuvamile ngoba ukugxila kwe-yttrium ngokwemvelo kuvame ukuphansi futhi akusetshenziswa kabanzi noma kuvezwe izidalwa zezinto eziphilayo ngokukhethekile kumanani akhona, futhi hhayi ukuba yinto edingekayo empilweni. Yize ingenawo imiphumela enobuthi ebonakalayo ezintweni eziphilayo ngaphansi kwezimo ezijwayelekile, ukuvezwa kwe-yttrium ephezulu kungadala ingozi yezempilo. Ngakho-ke, ucwaningo lwesayensi nokuqapha kusabalulekile emiphumeleni yezokuphepha neyokuphepha ye-YTtrium.
Ukusatshalaliswa kwe-ytttrium ngokwemvelo
I-YTTRIUM iyinto engavamile yomhlaba esatshalaliswa kabanzi ngokwemvelo, yize ayikho efomini le-aurel alement.
1. ISIQINISEKISO EMHLABENI WAMhlaba: Ubuningi be-YTTrium ekuqothulweni komhlaba buphansi, ngokuhlushwa okumaphakathi okungama-33 mg / kg. Lokhu kwenza i-ytttrium eyodwa yezinto ezingandile.
I-YTtrium ikhona ikakhulukazi ngesimo samaminerali, imvamisa kanye nezinye izinto zomhlaba ezingandile. Amanye amaminerali amakhulu we-yttrium afaka i-ytttrium iron garnet (yig) ne-ytttrium oxalate (Y2 (C2O4) 3).
2 Amanye amadiphozi amakhulu we-YTtrium angatholakala ezifundeni ezilandelayo: I-Australia, China, United States, eRussia, eCanada, i-Scandinavia, uma i-yttrium ore imbiwe, ukucubungula amakhemikhali lapho kudingeka khona ukukhipha i-yttrium. Lokhu kuvame ukubandakanya izinqubo zokuhlukaniswa kwe-acid kanye nezinqubo zokuhlukaniswa ngamakhemikhali ukuthola i-ytttrium ehlanzekile ephezulu.
Kubalulekile ukuqaphela ukuthi izinto zomhlaba ezingandile ezinjenge-ytttrium azivamisile ukuba zibe khona ngohlobo lwezinto ezihlanzekile, kepha zixubene nezinye izinto zomhlaba ezingandile. Ngakho-ke, ukukhishwa kwe-ytttrium ephakeme ye-ytttrium kudinga ukucubungula okuyinkimbinkimbi kwamakhemikhali nezinqubo zokwehlukanisa. Ngaphezu kwalokho, ukuhanjiswa kweizinto zomhlaba ezingavamileIkhawulelwe, ngakho-ke ukucatshangelwa kokuphathwa kwezinsizakusebenza nokusimama kwemvelo nakho kubalulekile.
Izimayini, ukukhishwa kanye nokubhema kwe-ytttrium element
I-YTTRIUM iyinto engavamile yomhlaba evame ukuba khona ngesimo se-ytttrium emsulwa, kepha ngesimo se-yttrium ore. Lokhu okulandelayo ukwethulwa okuningiliziwe kwenqubo yokushintsha kanye nokucwengeka kwe-YTtrium element:
1. Imayini ye-YTtrium Ore:
Ukuhlola: Okokuqala, onjiniyela bezokuma komhlaba kanye nonjiniyela bezimayini benza umsebenzi wokuhlola ukuthola amadiphozithi aqukethe i-yttrium. Lokhu kuvame ukufaka izifundo zemvelo, ukuhlola kwe-geophysical, nokuhlaziywa kwesampula. Imayini: Uma sekutholakale idiphozi equkethe i-ytttrium, i-ore imbiwe. Lezi zimali zivame ukufaka ama-ongide ama-oles anjenge-ytttrium iron garnet (yig) noma i-ytttrium oxalate (Y2 (C2O4) 3). Ukuchotshozwa kwe-ore: Ngemuva kokumbiwa phansi, i-ore imvamisa idinga ukuhlukaniswa ibe yizicucu ezincane zokucubungula okulandelayo.
2. Ukukhipha i-yttrium:I-Chemical Leaching: I-ore echotshoziwe ivame ukuthunyelwa ku-smelter, lapho i-yttrium ikhishwe khona ngokufakwa kwamakhemikhali. Le nqubo ivame ukusebenzisa ikhambi le-acidic leashing, njenge-sulfuric acid, ukuncibilikisa i-yttrium kusuka ku-ore. Ukuhlukaniswa: Uma ytttrium uchithwa, kuvame ukuxutshwa nezinye izinto zomhlaba ezingandile kanye nokungcola. Ukuze kukhishwe i-ytttrium of arpity ephakeme, inqubo yokuhlukanisa iyadingeka, imvamisa isebenzisa ukukhishwa kwe-solvent, i-ini ukushintshaniswa noma ezinye izindlela zamakhemikhali. I-Peripitation: I-YTtrium ihlukaniswe kwezinye izinto zomhlaba ezingandile ngokusebenzisa ukusabela okufanele kwamakhemikhali ukwakha amakhompiyutha ahlanzekile e-YTTRIUM. Ukomisa ne-Calloimed: Amakhompiyutha atholakala e-YTtrium ngokuvamile adinga ukomiswa futhi afakwe ku-Susa noma yimuphi umswakama osele kanye nokungcola ekugcineni ukuthola i-yttrium metal noma amakhompiyutha.
Izindlela zokutholwa ze-ytttrium
Izindlela ezivamile zokutholwa kwe-YTtrium ikakhulukazi zifaka phakathi i-atomic anlorption spectroscopy (AAS), ezihlanganisiwe ezihlanganisiwe ze-plasma mass spectrometry (i-ICP-MS), i-X-ray Fluorescence Spectroscopy (XRF), njll.
1. I-atomic anlorption spectroscopy (AAS):I-AAS iyindlela yokuhlaziya ejwayelekile esetshenziswa kakhulu efanelekile ukuthola okuqukethwe kwe-yttrium kusixazululo. Le ndlela isuselwa emcimbini wokumuncwa lapho into eqondiwe kwisampula idonsa ukukhanya kwe-wavelength ethile. Okokuqala, isampula liguqulwa libe yifomu elilinganiswayo ngezinyathelo zokuhlekisa ezinjengokuhlanganiswa kwegesi kanye nokomisa okushisa okuphezulu. Ngemuva kwalokho, ukukhanya okuhambelana ne-wavelength yento eqondiwe kudluliswa kwisampula, ubukhulu bokukhanya obudonswe yisampula bulinganiswa, futhi okuqukethwe kwe-yttrium kusampula kubalwa ngokuqhathanisa ne-yttrium solution evamile yokuhlushwa okujwayelekile.
2I-ICP-MS iyindlela ehlaziya ebucayi kakhulu yokuhlaziya efanelekile ukuthola okuqukethwe kwe-Yttrium kumasampula aqinile aqinile. Le ndlela iguqula isampula ibe izinhlayiya ezikhokhisiwe bese isebenzisa i-mass spectrometer yokuhlaziywa kwabantu abaningi. I-ICP-MS inebanga elibanzi lokuthola nokulungiswa okuphezulu, futhi linganquma okuqukethwe kwezinto eziningi ngasikhathi sinye. Ngokutholwa kwe-ytttrium, i-ICP-MS inganikeza imikhawulo ephansi kakhulu yokutholwa nokunemba okuphezulu.
3. I-X-ray Foontencence Spectrometry (XRF):I-XRF yindlela yokuhlaziya engeyona eyonakalisayo efanelekile ukunqunywa kokuqukethwe kwe-ytttrium kumasampula aqinile noketshezi. Le ndlela inquma okuqukethwe kwento ngokulwa kabusha ingaphezulu kwesampula nge-X-rays kanye nokulinganisa ubukhulu bokuqina kwe-fluorescence spectrum kusampula. I-XRF inezinzuzo zesivinini esisheshayo, ukusebenza okulula, kanye nekhono lokunquma izinto eziningi ngasikhathi sinye. Kodwa-ke, i-XRF ingaphazanyiswa ekuhlaziyweni kwe-YtTrium ephansi ye-YTTRIUM, okuholela emaphutheni amakhulu.
I-4.I-plasma ehlanganisiwe yokuphuma ye-plasma yokuphuma i-plasma i-spectrometry iyindlela ebucayi kakhulu futhi ekhethiwe yokuhlaziya ehlaziya ukuhlaziya okuningi. Izwakalisa isampula futhi ihlele i-plasma ukukala i-wavelength ethile kanye namandla of ytttriumukuphuma ku-spectrometer. Ngaphezu kwezindlela ezingenhla, kunezinye izindlela ezisetshenziswa kakhulu zokutholwa kwe-ytttrium, kufaka phakathi indlela ye-electrochemical, njll. Ukukhethwa kwendlela efanelekile yokutholwa kuncike ezintweni ezifana nezakhiwo zesampula ukuqinisekisa ukunemba nemiphumela yokulinganisa.
Ukusetshenziswa okukhethekile kwendlela yokuvumela i-atomic atomic
Ekulinganisweni kwe-element, okuhlanganiswe ngokungenisa i-plasma mass spectrometry (I-ICP-MS) inqubo yokuhlaziya ebucayi kakhulu futhi ehlukahlukene, evame ukusetshenziselwa ukuthola izici, kufaka phakathi i-yttrium. Lokhu okulandelayo inqubo eningiliziwe yokuhlola i-YTtrium ku-ICP-MS:
1. Ukulungiswa kwesampula:
Isampula ngokuvamile idinga ukuchithwa noma ukuhlakazwa kwifomu eliwuketshezi lokuhlaziywa kwe-ICP-MS. Lokhu kungenziwa ngokuchithwa kwamakhemikhali, ukufudumeza ukugaya noma ezinye izindlela zokulungiselela ezifanele.
Ukulungiswa kwesampula kudinga izimo ezihlanzekile kakhulu ukuvikela ukungcoliswa yinoma yiziphi izinto zangaphandle. Ilabhorethri kufanele ithathe izinyathelo ezidingekayo zokugwema ukungcoliswa kwesampula.
2 Isizukulwane se-ICP:
I-ICP yenziwa ngethula i-argon noma i-argon-oxygen igesi ehlanganisiwe kwithoshi elivaliwe le-quartz plasma. Ukuhlanganiswa okuyisisekelo okuphezulu kukhiqiza ilangabi elinamandla le-plasma, okuyiphuzu lokuqala lokuhlaziywa.
Izinga lokushisa le-plasma licishe libe ngu-8000 kuya ku-10000 degrees Celsius, okuphezulu ngokwanele ukuguqula izinto ekhasini le-Ionic State.
3. I-ionization kanye nokwehlukana:Lapho isampula lingena kwi-plasma, izakhi ezikulo ziyi-Ionosed. Lokhu kusho ukuthi ama-athomu alahlekelwe yi-elektroni elilodwa noma amaningi, akha ama-ion akhishwe. I-ICP-MS isebenzisa i-mass spectrometer ukuhlukanisa i-ion yezinto ezahlukene, imvamisa ngesilinganiso sokushaja (m / z). Lokhu kuvumela ama-ion wezinto ezahlukahlukene ukuthi zihlukaniswe futhi zihlaziywe kamuva.
4. I-Mass Spectrometry:Ama-ion ahlukanisiwe afaka i-mass spectrometer, imvamisa i-quadrupole mass spectrometer noma i-magnetic scan spectrometer. E-Mass Spectrometer, i-i-ion yezinto ezahlukahlukene zihlukaniswe futhi zitholwa ngokwesilinganiso sayo sokukhokhisa. Lokhu kuvumela ukuba khona kanye nokuhlushwa kwento ngayinye okufanele kunqunywe. Enye yezinzuzo ze-Plasma Mass spectrometry ngokungathandeki kuyisixazululo sayo esiphakeme, esikwenza sikwazi ukubona izinto eziningi ngasikhathi sinye.
5. Ukucutshungulwa kwedatha:Imininingwane ekhishwe yi-ICP-MS ngokuvamile idinga ukucutshungulwa futhi ihlaziywe ukuthola ukugxila kwezinto kusampula. Lokhu kufaka ukuqhathanisa isiginali yokutholwa kumazinga okugxila ezaziwayo, nokwenza ukulungiswa nokulungiswa.
6 Umbiko Wemiphumela:Umphumela wokugcina wethulwa njengephesenti lokuhlushwa noma amaphesenti amaningi wento. Le miphumela ingasetshenziswa ezinhlobonhlobo zezicelo, kufaka phakathi isayensi yezwe, ukuhlaziya imvelo, ukuhlolwa kokudla, ucwaningo lwezokwelapha, njll.
I-ICP-MS iyindlela enembile futhi ebucayi inqubo elungele ukuhlaziywa kwezinto eziningi, kufaka phakathi i-YTTRIUM. Kodwa-ke, kudinga ukuthunyelwa okuyinkimbinkimbi nobuchwepheshe, ngakho-ke kuvame ukwenziwa ngelebhu noma esikhungweni sokuhlaziywa kobungcweti. Emsebenzini wangempela, kuyadingeka ukuthi ukhethe indlela efanelekile yokulinganisa ngokuya ngezidingo ezithile zesayithi. Lezi zindlela zisetshenziswa kabanzi ekuhlaziyweni nasekutholeni i-yterterbium ezindaweni zokucwaninga kanye nezimboni.
Ngemuva kokufingqa okungenhla, singaphetha ngokuthi i-yttrium iyinto ethokozisayo yamakhemikhali enezinto ezihlukile zomzimba nangamakhemikhali, ezibaluleke kakhulu ocwaningweni lwesayensi kanye nezinkundla zohlelo lokusebenza. Yize senze isinqumo esithile ekuqondeni kwethu, kusenemibuzo eminingi edinga ucwaningo olwengeziwe nokuhlola okwengeziwe. Ngiyethemba ukuthi ukwethulwa kwethu kungasiza abafundi baqonde kangcono le nto ethokozisayo futhi bagqugquzele uthando lwawo wonke umuntu ngesayensi nentshisekelo ekuhlolweni.
Ngeminye imininingwane plsXhumana nathiNgezansi:
I-TEL & Whats: 008613524231522
Email:Sales@shxlchem.com
Isikhathi sePosi: Nov-28-2024