Cobolt coode co3o4 ntụ ntụ

Nkowa
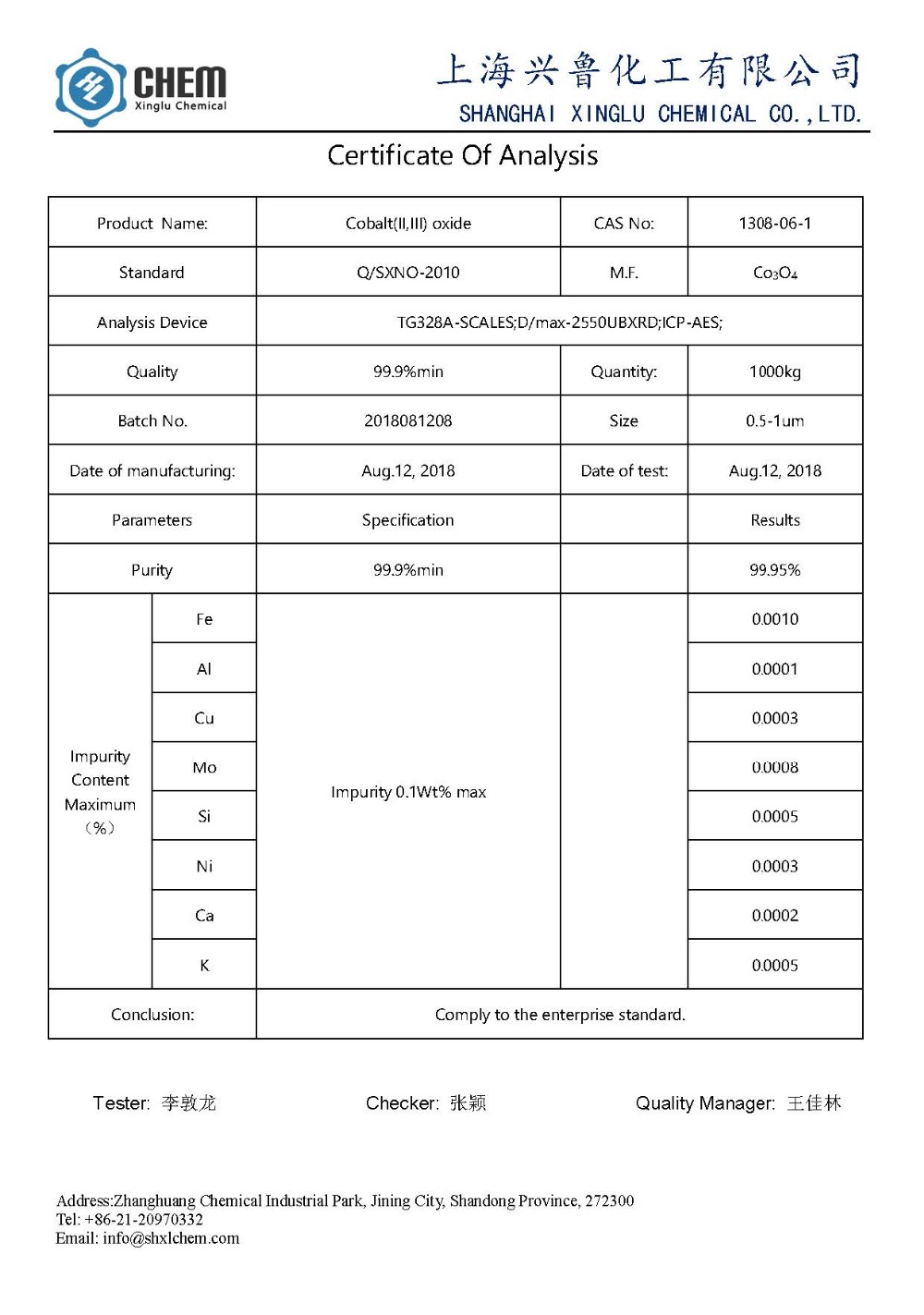
1.Name: NanoCobaltlexideCo3o4 ntụ
2.Pist: 99.9% min
3.pphitaacne: ntụ ntụ ojii
4.Particle nha: 50nm
5.Ssa: 30-80 m2 / g
Olu:
Nkpughere ikuku, ọ dị mfe ịnabata mmiri, ma ọ naghị emepụta ogige mmiri. Ọ bụ soluble na nitric acid. Mgbe a ga-agbada na-ewe iwe ruo elekere iri na abụọ, a ga-agbada Nano-carblidexi. Na hydrogen ire ọkụ, Nano-Cobalt oxide ruo 900 oc, a ga-agbanwe ya n'ime ọla. COBACK (II, III) oxide bụ kemịkalụ na usoro Co3O4. Ọ bụ nwa ojii siri ike, yana ọnụahịa agwakọta, nke nwere ma co (iI) na co (III). Enwere ike ịhazi ya dịka coiicoii2O4 ma ọ bụ coo.co2o3. Cobtide (II) oxide, COO, na-atụgharị ka C3O4 ma ọ bụrụ na ọ kpoo ọkụ ruo ihe dị ka 600-700 Celsius C na ikuku. N'elu 900 Celsius C, COO kwụ chịm.
Ngwa:
Mkpokọta, ceramictors, ceramics na ubi ndị ọzọ dị ka ihe dị mkpa na-aga; Dị ka onye na-ahụ maka ihe na-eweta ya na ihe eletriki na-arụ ọrụ; Maka iko, mpempe poselain na ụcha; Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na). Mkparịta ụka ndị okenye na akụrụngwa ndị ọzọ; Karọdi; Okpomọkụ na gas; Maka ụlọ ọrụ semiconductor, caramics elektrik, lithium ion batrị na-elekwasị anya, ihe ndọta; Ngwaọrụ electromic; Enamels; Na-egweri wiil; Heteyouuss na-enweghị atụ; Anyanwụ na-aga n'ihu ....
Asambodo:

Ihe anyi puru inye: