Cobalt Oxide Co3O4 phofo

TLHOKOMELISO
1.NANA: nanoCobalt OxideCo3O4 phofo
2. Khalefo: 99.9,9% Min
3. 3.PECIPECORE: Nyow phofo e ntšo
Meetso ea 4.Partle: 50nm
5.ssa: 30-80 m2 / g
Thepa:
Ho pepesehela moea, ho bonolo ho amohela mongobo, empa ha e hlahise metsoako ea metsi. E qhibiliha nitric acid. Ha a futhumetse ho kaholimo ho 1200 Oc, Nano-Cobalt oxide e tla robeha ho sub-cobate. Lelapeng la hydrojene, oxide e tuka ho 900 Oc, e tla fetoloa ka cobaltit ea tšepe. Cobalt (II, III) oxide ke motsoako oa lik'hemik'hale le formula co34. Ke moqhobi ea sootho, le valence e kopaneng, e nang le co (II) le co (iii) oxidation. E ka bokelloa e le Coiicoii2o4 kapa coo.co2o3. Cobalt (ii) oxide, coo, e fetolela Co3O4 haeba e futhumetse ho pota 600-7 ° C ka moea. Kaholimo ho 900 ° C, C, C, e tsitsitse.
Kopo:
Catalysis, adconductos le masimo a mang e le lisebelisoa tsa bohlokoa tse sa tsotelleng; Joalo ka ha li-catalyst le carriers carer le lisebelisoa tsa elektrode tse sebetsang; Bakeng sa khalase ea khalase, li-coloration le mebala ea porcelaine le mebala; Liipome tsa indasteri ea lik'hemik'hale litsitsa tsa lik'hemik'hale le katsene ea maiketsetso; Li-Goggles tse phahameng le lisebelisoa tse ling tsa filthara; Carbide; Methapo ea mocheso le khase; Bakeng sa indasteri ea semiconductor, li-ceramiccctor tsa elektroniki, lithium ion beri lisebelisoa tsa elektrode, lisebelisoa tsa makenete; Lisebelisoa tsa Electrochromic; Menahano; Mabili a ho silila; Heterogenesiof catalysts; Solar Energer e kenella ....
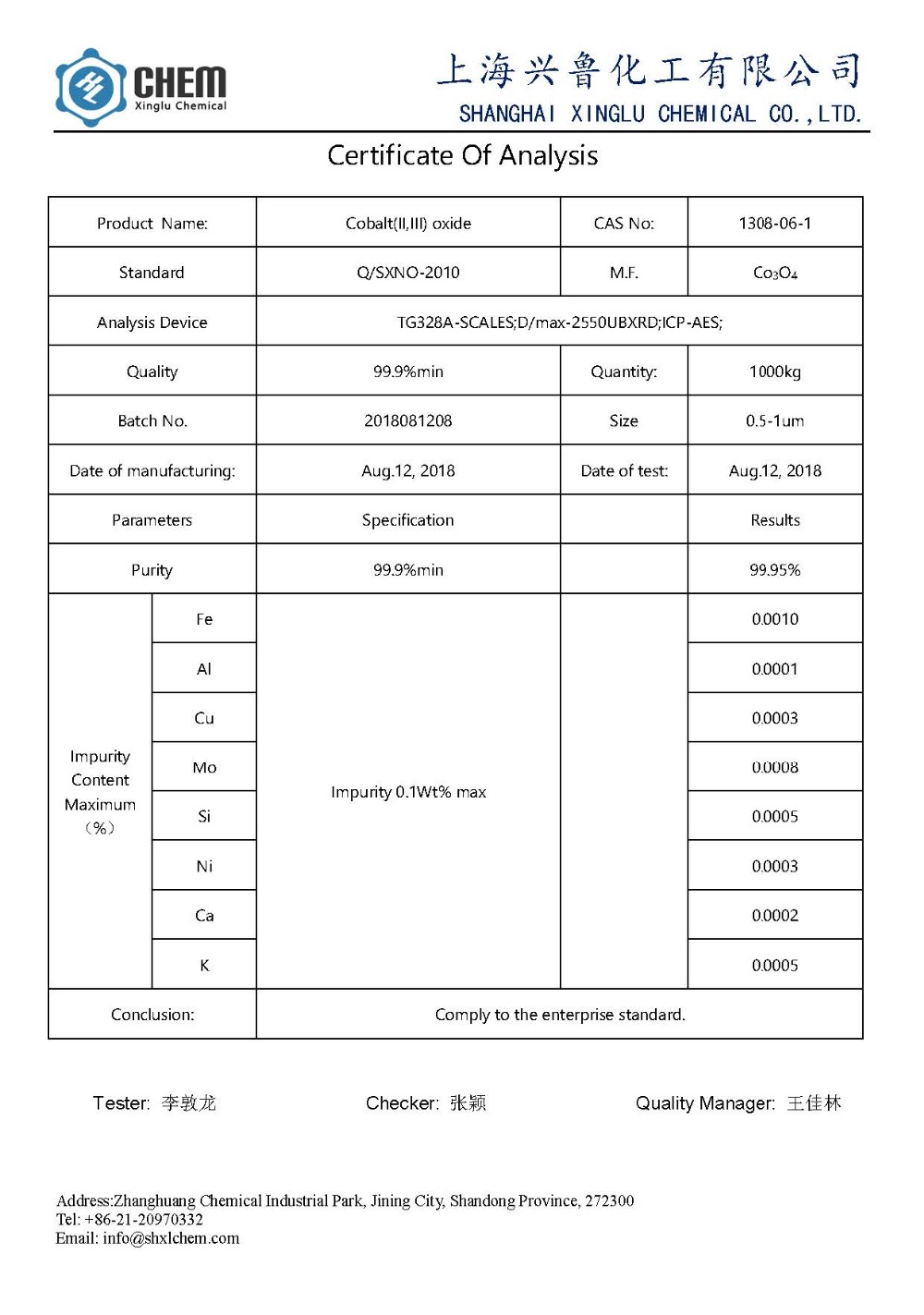
Setifikeiti:

Seo re ka se Fanang ka Sona: